Difference between revisions of "Chemical Reaction"
(→Examples) |
|||
| Line 28: | Line 28: | ||
|[[File:RearrangeAtoms1.png|center|200px]] | |[[File:RearrangeAtoms1.png|center|200px]] | ||
|- | |- | ||
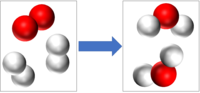
| − | | style="height:20px; width:200px; text-align:center;" |2H<sub>2</sub>+O<sub>2</sub> → 2H<sub>2</sub>O | + | | style="height:20px; width:200px; text-align:center;" |2H<sub>2</sub> + O<sub>2</sub> → 2H<sub>2</sub>O |
|} | |} | ||
| Line 35: | Line 35: | ||
|[[File:RearrangeAtoms2.png|center|200px]] | |[[File:RearrangeAtoms2.png|center|200px]] | ||
|- | |- | ||
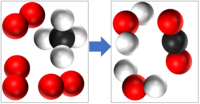
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |CH<sub>4</sub> + 2O<sub>2</sub> → 2H<sub>2</sub>O + CO<sub>2</sub> |
|} | |} | ||
Revision as of 18:46, 23 September 2018
Contents
Key Stage 2
Meaning
A chemical reaction is when one substance changes into another.
About Chemical Reactions
- Most chemical reactions are irreversible.
- Chemical reactions usually cause a change in temperature.
- Chemical reactions you should know are:
Key Stage 3
Meaning
A Chemical Reaction is when the atoms in molecules break their bonds and form new bonds, usually with other atoms.
About Chemical Reactions
- Most chemical reactions are irreversible.
- In a chemical reaction the atoms rearrange to form a new substance.
- Chemical reactions often need some energy to start, like combustion where the fuel needs to be heated to start.
- If a chemical reaction gives off more energy than it needs to start then it is called an Exothermic Reaction. In these reactions the temperature increases.
- If a chemical reaction takes in more energy than it gives out then it is called an Endothermic Reaction.
In these reactions the temperature decreases.
Examples
| 2H2 + O2 → 2H2O |
| CH4 + 2O2 → 2H2O + CO2 |