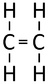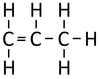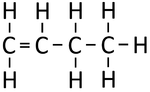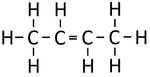Difference between revisions of "Alkene"
(→Examples) |
(→Key Stage 4) |
||
| Line 33: | Line 33: | ||
|[[File:StructuralDiagramPropene.png|center|100px]] | |[[File:StructuralDiagramPropene.png|center|100px]] | ||
|[[File:StructuralDiagramBut-1-ene.png|center|150px]] | |[[File:StructuralDiagramBut-1-ene.png|center|150px]] | ||
| − | |[[File:StructuralDiagramBut-2-ene.png|center| | + | |[[File:StructuralDiagramBut-2-ene.png|center|150px]] |
|- | |- | ||
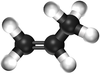
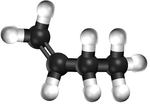
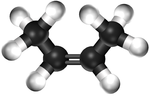
| style="height:20px; width:200px; text-align:center;" |[[Ball and Stick Model]] | | style="height:20px; width:200px; text-align:center;" |[[Ball and Stick Model]] | ||
| Line 39: | Line 39: | ||
|[[File:BallandStickPropene.png|center|100px]] | |[[File:BallandStickPropene.png|center|100px]] | ||
|[[File:BallandStickBut-1-ene.png|center|150px]] | |[[File:BallandStickBut-1-ene.png|center|150px]] | ||
| − | |[[File:BallandStickBut-2-ene.png|center| | + | |[[File:BallandStickBut-2-ene.png|center|150px]] |
|} | |} | ||
Revision as of 13:28, 17 January 2019
Key Stage 4
Meaning
Alkenes are hydrocarbon compounds with one double bond and the general formula; CnH2n
About Alkenes
- Alkenes are a homologous series of hydrocarbon compounds.
- The functional group of the Alkenes is the double bonds between the Carbon atoms.
- Alkenes are long chains of Carbon atoms covalently bonded together with double and single bonds and Hydrogen atoms taking the remaining bonds.
Examples
| Ethene | Propene | But-1-ene | But-2-ene | |
| Chemical Formula (CnH2n) | C2H4 | C3H6 | C4H8 | C4H8 |
| Structural Formula | CH2CH2 | CH2CHCH3 | CH2CHCH2CH3 | CH3CHCHCH3 |
| Structural Diagram | ||||
| Ball and Stick Model |