Key Stage 3
Meaning
The Ice-Water Anomaly is the observation that water in its solid state is less dense than water in its liquid state.
About the Ice-Water Anomaly
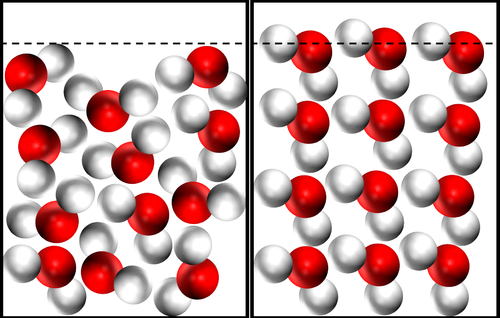
- For most substances the solid state is more dense than the liquid state. However, this is not the case for water.
- In liquid water the molecules are randomly arranged and close together. In solid water the molecules align with their Hydrogen atoms touching the Oxygen atoms of adjacent molecules.
| The same number of molecules takes up a larger volume in solid water, so Ice is less dense than liquid water. |