Contents
Key Stage 3
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
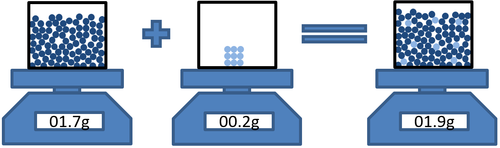
- In dissolving conservation of mass means that the mass of the solvent and the mass of the solute can be added to find the mass of the solution.
| Masssolvent + Masssolute = Masssolution |
- In a chemical reaction conservation of mass means that the same atoms which made up the reactants must make up the products. So the atoms are not created or destroyed in a chemical reaction, they are just rearranged.
|
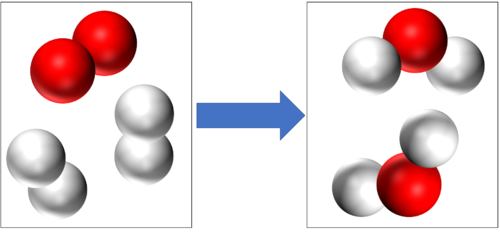
Conservation of mass tells us that if there are 4 Hydrogen atoms and 2 Oxygen atoms at the start of this reaction then there will be the end of the reaction 4 Hydrogen atoms and 2 Oxygen atoms at the end of the reaction. |
|
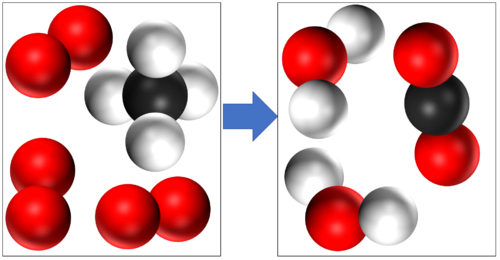
In this reaction you can see that mass is conserved because there are 4 Hydrogen atoms, 4 Oxygen atoms and 1 Carbon atom in the reactants and all the same atoms are found in the products. |
Key Stage 4
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
- In a chemical reaction law of conservation of mass indicates that the total mass of the products is the same as the total mass of the reactants.
Examples
Methane + Oxygen → Water + Carbon Dioxide CH4 + 2O2 → 2H2O + CO2