Endothermic
Revision as of 18:13, 29 September 2018 by NRJC (talk | contribs) (Created page with "==Key Stage 3== ===Meaning=== An endothermic process is one takes in energy. This usually causes surroundings to decrease in temperature. ===About Exothermic Proc...")
Key Stage 3
Meaning
An endothermic process is one takes in energy. This usually causes surroundings to decrease in temperature.
About Exothermic Processes
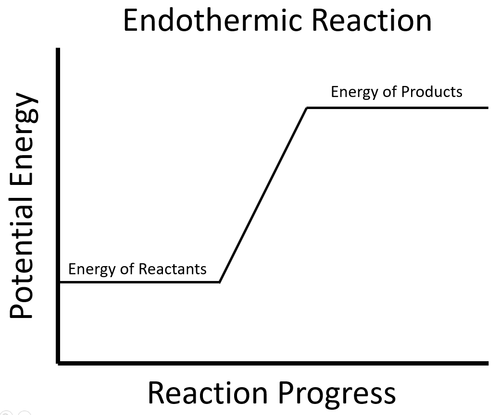
- A few chemical reactions are endothermic with means they need energy from the environment and this is observed by a decrease in temperature.
| The energy stored in the reactants less than the energy stored in the products. This means the reaction must take in energy from the environment which causes the surroundings to decrease in temperature. |
- Melting, Evaporating and Subliming are endothermic changes because they need energy to happen. The material has more energy at the end than it did the beginning. However, there is usually no decrease in temperature because the material is usually being heated to change state.
- Dissolving is an endothermic process as energy is needed to break the bonds in the solid solute to dissolve. This causes the solution to decrease in temperature while the solute is dissolving.