Difference between revisions of "Gas"
(Created page with "A state of matter in which the particles are separated by large distances and can move freely.") |
|||
| (22 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | ==Key Stage 2== | |
| + | ===Meaning=== | ||
| + | '''Gas''' is a [[State of Matter|state of matter]] that can change size and shape to fit any container. | ||
| + | |||
| + | ===About Gases=== | ||
| + | : Most '''gases''' are invisible but we can feel them. | ||
| + | : When the [[air]] moves we call it the wind. | ||
| + | {| class="wikitable" | ||
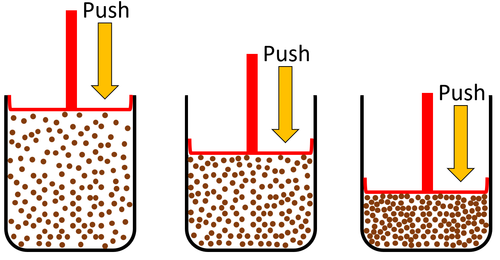
| + | |+ Gases are a '''state of matter''' that: | ||
| + | |- | ||
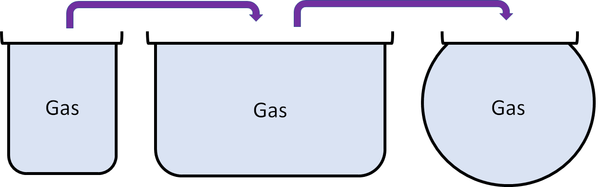
| + | |[[File:GasPour.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:600px; text-align:left;" | | ||
| + | *Cannot hold their shape. | ||
| + | *Fit the shape of their container. | ||
| + | *Can be poured and will flow. | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
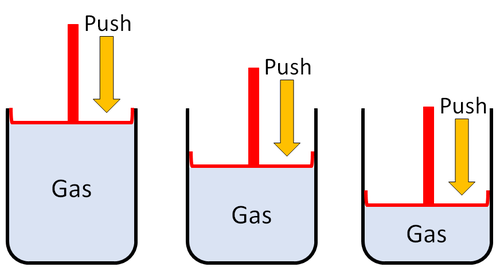
| + | |[[File:GasSquash.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:left;" |'''Gases''' can be squashed into a smaller size. | ||
| + | |} | ||
| + | |||
| + | Examples of gas [[Material|materials]]: | ||
| + | *Air (A mixture of gases, mostly nitrogen and oxygen) | ||
| + | *Steam | ||
| + | |||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
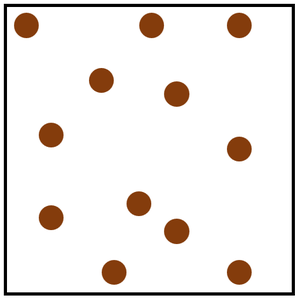
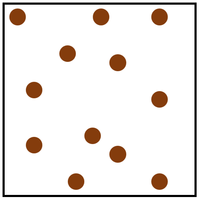
| + | [[File:ParticleModelGas.png|right|300px|thumb|The [[Particle Model]] of a [[gas]].]] | ||
| + | [[Gas]] is a [[State of Matter]] in which the [[Particle|particles]] are separated by large distances and can move freely. | ||
| + | |||
| + | ===About Gases=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
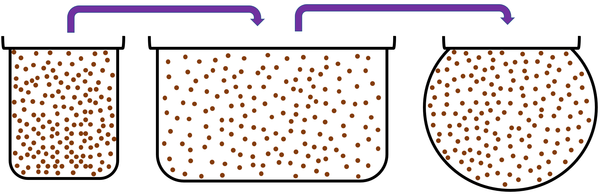
| + | |[[File:ParticleModelGasPour.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:600px; text-align:left;" | | ||
| + | *[[Gas]]es cannot hold their shape because the particles are free to move. | ||
| + | *[[Gas]]es fit the shape of their containers because the particles are free to move. | ||
| + | *A [[gas]] can be poured and will flow because the particles are free to move. | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:ParticleModelGasSquash.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:left;" |'''Gas''' can be squashed into a smaller size because the [[particle]]s are spread apart. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 4 Foundation== | ||
| + | ===Meaning=== | ||
| + | [[Gas]] is a [[State of Matter]] in which the [[Particle|particles]] are separated by large distances and can move freely. | ||
| + | |||
| + | ===About Gases=== | ||
| + | : When a [[substance]] is in its [[gas]]eous [[State of Matter|state]] it is always less [[Density|dense]] than in its [[liquid]] or [[solid]] [[State of Matter|state]] due to the [[particle]]s in a [[gas]] being spread far apart from each other. | ||
| + | : A [[substance]] which is [[gas]]eous at [[Room Temperature|room temperature]] has a smaller [[force]] of [[attraction]] between [[particle]]s than a [[substance]] which is [[liquid]] or [[solid]] at [[Room Temperature|room temperature]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+Gases | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Diagram''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Arrangement''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Property''' | ||
| + | |- | ||
| + | |rowspan="6"|[[File:ParticleModelGas.png|center|200px]] | ||
| + | |rowspan="3"|[[Particle]]s are free to move in all directions. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Gas]]es fit the size of their container. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Gas]]es fit the shape of their container. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Convection]] happens most easily in [[gas]]es. | ||
| + | |- | ||
| + | |rowspan="3"|[[Particle]]s are spread apart. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Gas]]es can be [[compressed]] into a smaller [[Volume (Space)|volume]]. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Sound]] passes through [[gas]]es slower than [[liquid]]s and [[solid]]s. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] is very poor in a [[gas]]es. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 4 Higher== | ||
| + | ===Molar Gas Volume=== | ||
| + | : At [[Standard Temperature and Pressure]] (20°C and 101,000Pa) the [[Volume (Space)|volume]] of one [[mole]] of [[gas]] is 24 dm<sup>3</sup>. | ||
| + | : The [[Volume (Space)|volume]] of [[gas]] required or [[product|produced]] in a [[Chemical Reaction|chemical reaction]] can be calculated from the [[mass]] of the [[gas]] and its [[Relative Formula Mass]]. | ||
| + | ====Example Calculations==== | ||
| + | {| class="wikitable" | ||
| + | |+ Finding the Volume of Gas | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''Calculate the [[Volume (Space)|volume]] of 4g of [[Hydrogen]] [[gas]].''' | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''Calculate the [[Volume (Space)|volume]] of 8g of [[Oxygen]] [[gas]].''' | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''Calculate the [[Volume (Space)|volume]] of 20g of [[Methane]] [[gas]].''' | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |1.'''Find the [[Relative Formula Mass]] of the [[gas]].''' | ||
| + | |||
| + | [[Chemical Formula]] of [[Hydrogen]] = H<sub>2</sub> | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Hydrogen]] = 1g | ||
| + | |||
| + | [[Relative Formula Mass]] of H<sub>2</sub> = 2g | ||
| + | |||
| + | | style="height:20px; width:250px; text-align:center;" |1.'''Find the [[Relative Formula Mass]] of the [[gas]].''' | ||
| + | |||
| + | [[Chemical Formula]] of [[Oxygen]] = O<sub>2</sub> | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Oxygen]] = 16g | ||
| + | |||
| + | [[Relative Formula Mass]] of O<sub>2</sub> = 32g | ||
| + | | style="height:20px; width:250px; text-align:center;" |1.'''Find the [[Relative Formula Mass]] of the [[gas]].''' | ||
| + | |||
| + | [[Chemical Formula]] of [[Methane]] = CH<sub>4</sub> | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Hydrogen]] = 1g | ||
| + | |||
| + | |||
| + | [[Relative Atomic Mass]] of [[Carbon]] = 12g | ||
| + | |||
| + | [[Relative Formula Mass]] of CH<sub>4</sub> = 16g | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''2. Calculate the number of [[mole]]s of [[gas]].''' | ||
| + | |||
| + | 1 [[mole]] = 2g | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{4}{2}</math> | ||
| + | |||
| + | No. [[Mole]]s = 2mol | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''2. Calculate the number of [[mole]]s of [[gas]].''' | ||
| + | |||
| + | 1 [[mole]] = 32g | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{8}{32}</math> | ||
| + | |||
| + | No. [[Mole]]s = 0.25 mol | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''2. Calculate the number of [[mole]]s of [[gas]].''' | ||
| + | |||
| + | 1 [[mole]] = 16g | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{20}{16}</math> | ||
| + | |||
| + | No. [[Mole]]s = 1.25 mol | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''3. Find the [[Volume (Space)|volume]] in dm<sup>3</sup>.''' | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (number of moles) | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (2) | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 48dm<sup>3</sup> | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''3. Find the [[Volume (Space)|volume]] in dm<sup>3</sup>.''' | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (number of moles) | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (0.25) | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 6dm<sup>3</sup> | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''3. Find the [[Volume (Space)|volume]] in dm<sup>3</sup>.''' | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (number of moles) | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (1.25) | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 30dm<sup>3</sup> | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ Finding the Mass of Gas from the Volume | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''Calculate the [[mass]] of 12dm<sup>3</sup> of [[Hydrogen]] [[gas]].''' | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''Calculate the [[mass]] of 36dm<sup>3</sup> of [[Nitrogen]] [[gas]].''' | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''Calculate the [[mass]] of 18dm<sup>3</sup> of [[Ethane]] [[gas]].''' | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |1.'''Find the [[Relative Formula Mass]] of the [[gas]].''' | ||
| + | |||
| + | [[Chemical Formula]] of [[Hydrogen]] = H<sub>2</sub> | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Hydrogen]] = 1g | ||
| + | |||
| + | [[Relative Formula Mass]] of H<sub>2</sub> = 2g | ||
| + | |||
| + | | style="height:20px; width:250px; text-align:center;" |1.'''Find the [[Relative Formula Mass]] of the [[gas]].''' | ||
| + | |||
| + | [[Chemical Formula]] of [[Nitrogen]] = N<sub>2</sub> | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Nitrogen]] = 14g | ||
| + | |||
| + | [[Relative Formula Mass]] of N<sub>2</sub> = 28g | ||
| + | | style="height:20px; width:250px; text-align:center;" |1.'''Find the [[Relative Formula Mass]] of the [[gas]].''' | ||
| + | |||
| + | [[Chemical Formula]] of [[Ethane]] = C<sub>2</sub>H<sub>6</sub> | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Hydrogen]] = 1g | ||
| + | |||
| + | [[Relative Atomic Mass]] of [[Carbon]] = 12g | ||
| + | |||
| + | [[Relative Formula Mass]] of C<sub>2</sub>H<sub>6</sub> = 30g | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''2. Calculate the number of [[mole]]s of [[gas]].''' | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (number of moles) | ||
| + | |||
| + | 12 = 24 x (number of moles) | ||
| + | |||
| + | Number of moles = 12/24 | ||
| + | |||
| + | Number of moles = 0.5 | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''2. Calculate the number of [[mole]]s of [[gas]].''' | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (number of moles) | ||
| + | |||
| + | 36 = 24 x (number of moles) | ||
| + | |||
| + | Number of moles = 36/24 | ||
| + | |||
| + | Number of moles = 1.5 | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''2. Calculate the number of [[mole]]s of [[gas]].''' | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 24 x (number of moles) | ||
| + | |||
| + | 12 = 24 x (number of moles) | ||
| + | |||
| + | Number of moles = 18/24 | ||
| + | |||
| + | Number of moles = 0.75 | ||
| + | |- | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''3. Find the [[mass]] of [[gas]] from the number of [[mole]]s.''' | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | 0.5 = <math>\frac{Mass}{2}</math> | ||
| + | |||
| + | Mass = <math>0.5\times2</math> | ||
| + | |||
| + | Mass = 1g | ||
| + | [[Volume (Space)|Volume]] = 48dm<sup>3</sup> | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''3. Find the [[mass]] of [[gas]] from the number of [[mole]]s.''' | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | 1.5 = <math>\frac{Mass}{28}</math> | ||
| + | |||
| + | Mass = <math>1.5\times28</math> | ||
| + | |||
| + | Mass = 42g | ||
| + | | style="height:20px; width:250px; text-align:center;" |'''3. Find the [[mass]] of [[gas]] from the number of [[mole]]s.''' | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | 0.75 = <math>\frac{Mass}{30}</math> | ||
| + | |||
| + | Mass = <math>0.75\times30</math> | ||
| + | |||
| + | Mass = 22.5g | ||
| + | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Gas, pages 13, 34-5, 82-5, 96-101, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Gas; pressure, pages 82-5, 96-9, 257, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Gas; volume, page 98, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d137 ''Gases pages 36, 37, 46, 107, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Gases, page 107, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Gases, page 71, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d254 ''Gases, page 88, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Gases, page 97, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Gases, pages 100, 101, 124, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Gases, pages 164-5, 323, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Gases, pages 6, 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Gases, pages 78-79, 83, 86-89, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Gases, pages 98, 99, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Gases; analysis, pages 184-185, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Gases; atmosphere, pages 194-205, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Gases; density of, page 323, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09923 ''Gases; gas pressure, page 193, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac211 ''Gases; gas pressure, page 41, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09924 ''Gases; gas syringes, pages 140, 232, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Gases; greenhouse effect, pages 198-202, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Gases; Greenhouse gases, pages 170-3, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Gases; identification of, page 207, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Gases; Identification of, pages 160, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Gases; In the atmosphere, pages 167, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09925 ''Gases; measuring volumes, pages 140, 232, 237, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09926 ''Gases; natural gas, page 178, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac212 ''Gases; natural gas, pages 18, 21, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Gases; natural gas, pages 45, 46 54, 56, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Gases; natural gas, pages 47, 48, 56, 58, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Gases; particle model of, pages 330-1, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Gases; particle model of, pages 78-80, 85-6, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Gases; particle motion, page 104, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09927 ''Gases; particle motion, page 193, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Gases; particle motion, pages 114, 115, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac213 ''Gases; particle motion, pages 38, 41, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Gases; pressure, page 104, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Gases; pressure, page 36, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Gases; pressure, pages 114, 115, 169, 171, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Gases; states of matter, pages 106, 107, 110-112, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09928 ''Gases; states of matter, pages 121, 122, 193, 195, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac214 ''Gases; states of matter, pages 38-40, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Gases; states of matter, pages 96, 97, 100-102, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09929 ''Gases; tests for, page 153, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Gases; Volume and mass measurement, pages 120, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Gases; volume, pages 78-79, 129, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Gases, molar volumes of, pages 118-119, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Gases, page 300, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Gases, pages 34, 35, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Gases, pages 96, 98, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Gases, pages 97, 98, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Gases; doing work, page 312, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Gases; pressure, page 424, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Gases; pressure, pages 192, 194-195, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Gases; pressure, pages 310-312, 319, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Gases; temperature, page 192, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Gases; temperature, page 424, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Gases; volume, pages 194-195, GCSE Physics, Pearson Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Gases, page 12, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases, page 18, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945687/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945687&linkCode=as2&tag=nrjc-21&linkId=9a598e52189317a20311d7a632747bc9 ''Gases, pages 14, 17, 18, Gateway GCSE Physics; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Gases, pages 82, 152, 155, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; atmosphere, pages 252-257, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; calculating amounts, pages 170-171, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; changing state, pages 76-77, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Gases; collecting, pages 128, 222, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; collection, pages 265, 267, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; detection, pages 146-147, 265, 267, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945687/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945687&linkCode=as2&tag=nrjc-21&linkId=9a598e52189317a20311d7a632747bc9 ''Gases; pressure in, pages 17, 18, Gateway GCSE Physics; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; pressure, page 179, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; refined crude oil, page 239, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Gases; volume, pages 170-171, 174, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 11:37, 11 December 2019
Contents
Key Stage 2
Meaning
Gas is a state of matter that can change size and shape to fit any container.
About Gases
- Most gases are invisible but we can feel them.
- When the air moves we call it the wind.
|
| Gases can be squashed into a smaller size. |
Examples of gas materials:
- Air (A mixture of gases, mostly nitrogen and oxygen)
- Steam
Key Stage 3
Meaning
Gas is a State of Matter in which the particles are separated by large distances and can move freely.
About Gases
| Gas can be squashed into a smaller size because the particles are spread apart. |
Key Stage 4 Foundation
Meaning
Gas is a State of Matter in which the particles are separated by large distances and can move freely.
About Gases
- When a substance is in its gaseous state it is always less dense than in its liquid or solid state due to the particles in a gas being spread far apart from each other.
- A substance which is gaseous at room temperature has a smaller force of attraction between particles than a substance which is liquid or solid at room temperature.
| Particle Diagram | Particle Arrangement | Property |
| Particles are free to move in all directions. | Gases fit the size of their container. | |
| Gases fit the shape of their container. | ||
| Convection happens most easily in gases. | ||
| Particles are spread apart. | Gases can be compressed into a smaller volume. | |
| Sound passes through gases slower than liquids and solids. | ||
| Thermal Conduction is very poor in a gases. |
Key Stage 4 Higher
Molar Gas Volume
- At Standard Temperature and Pressure (20°C and 101,000Pa) the volume of one mole of gas is 24 dm3.
- The volume of gas required or produced in a chemical reaction can be calculated from the mass of the gas and its Relative Formula Mass.
Example Calculations
| Calculate the volume of 4g of Hydrogen gas. | Calculate the volume of 8g of Oxygen gas. | Calculate the volume of 20g of Methane gas. |
| 1.Find the Relative Formula Mass of the gas.
Chemical Formula of Hydrogen = H2 Relative Atomic Mass of Hydrogen = 1g Relative Formula Mass of H2 = 2g |
1.Find the Relative Formula Mass of the gas.
Chemical Formula of Oxygen = O2 Relative Atomic Mass of Oxygen = 16g Relative Formula Mass of O2 = 32g |
1.Find the Relative Formula Mass of the gas.
Chemical Formula of Methane = CH4 Relative Atomic Mass of Hydrogen = 1g
Relative Formula Mass of CH4 = 16g |
| 2. Calculate the number of moles of gas.
1 mole = 2g No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{4}{2}\) No. Moles = 2mol |
2. Calculate the number of moles of gas.
1 mole = 32g No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{8}{32}\) No. Moles = 0.25 mol |
2. Calculate the number of moles of gas.
1 mole = 16g No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{20}{16}\) No. Moles = 1.25 mol |
| 3. Find the volume in dm3.
Volume = 24 x (number of moles) Volume = 24 x (2) Volume = 48dm3 |
3. Find the volume in dm3.
Volume = 24 x (number of moles) Volume = 24 x (0.25) Volume = 6dm3 |
3. Find the volume in dm3.
Volume = 24 x (number of moles) Volume = 24 x (1.25) Volume = 30dm3 |
| Calculate the mass of 12dm3 of Hydrogen gas. | Calculate the mass of 36dm3 of Nitrogen gas. | Calculate the mass of 18dm3 of Ethane gas. |
| 1.Find the Relative Formula Mass of the gas.
Chemical Formula of Hydrogen = H2 Relative Atomic Mass of Hydrogen = 1g Relative Formula Mass of H2 = 2g |
1.Find the Relative Formula Mass of the gas.
Chemical Formula of Nitrogen = N2 Relative Atomic Mass of Nitrogen = 14g Relative Formula Mass of N2 = 28g |
1.Find the Relative Formula Mass of the gas.
Chemical Formula of Ethane = C2H6 Relative Atomic Mass of Hydrogen = 1g Relative Atomic Mass of Carbon = 12g Relative Formula Mass of C2H6 = 30g |
| 2. Calculate the number of moles of gas.
Volume = 24 x (number of moles) 12 = 24 x (number of moles) Number of moles = 12/24 Number of moles = 0.5 |
2. Calculate the number of moles of gas.
Volume = 24 x (number of moles) 36 = 24 x (number of moles) Number of moles = 36/24 Number of moles = 1.5 |
2. Calculate the number of moles of gas.
Volume = 24 x (number of moles) 12 = 24 x (number of moles) Number of moles = 18/24 Number of moles = 0.75 |
| 3. Find the mass of gas from the number of moles.
No. Moles = \(\frac{Mass}{M_r}\) 0.5 = \(\frac{Mass}{2}\) Mass = \(0.5\times2\) Mass = 1g Volume = 48dm3 |
3. Find the mass of gas from the number of moles.
No. Moles = \(\frac{Mass}{M_r}\) 1.5 = \(\frac{Mass}{28}\) Mass = \(1.5\times28\) Mass = 42g |
3. Find the mass of gas from the number of moles.
No. Moles = \(\frac{Mass}{M_r}\) 0.75 = \(\frac{Mass}{30}\) Mass = \(0.75\times30\) Mass = 22.5g |
References
AQA
- Gas, pages 13, 34-5, 82-5, 96-101, GCSE Physics; Student Book, Collins, AQA
- Gas; pressure, pages 82-5, 96-9, 257, GCSE Physics; Student Book, Collins, AQA
- Gas; volume, page 98, GCSE Physics; Student Book, Collins, AQA
- Gases pages 36, 37, 46, 107, GCSE Chemistry; The Revision Guide, CGP, AQA
- Gases, page 107, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Gases, page 71, GCSE Physics, Hodder, AQA
- Gases, page 88, GCSE Chemistry; The Revision Guide, CGP, AQA
- Gases, page 97, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Gases, pages 100, 101, 124, GCSE Chemistry, CGP, AQA
- Gases, pages 164-5, 323, GCSE Combined Science Trilogy 1, Hodder, AQA
- Gases, pages 6, 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Gases, pages 78-79, 83, 86-89, GCSE Physics; Third Edition, Oxford University Press, AQA
- Gases, pages 98, 99, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Gases; analysis, pages 184-185, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Gases; atmosphere, pages 194-205, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Gases; density of, page 323, GCSE Combined Science Trilogy 1, Hodder, AQA
- Gases; gas pressure, page 193, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; gas pressure, page 41, GCSE Physics; The Revision Guide, CGP, AQA
- Gases; gas syringes, pages 140, 232, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; greenhouse effect, pages 198-202, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Gases; Greenhouse gases, pages 170-3, GCSE Combined Science Trilogy 2, Hodder, AQA
- Gases; identification of, page 207, GCSE Chemistry, Hodder, AQA
- Gases; Identification of, pages 160, GCSE Combined Science Trilogy 2, Hodder, AQA
- Gases; In the atmosphere, pages 167, GCSE Combined Science Trilogy 2, Hodder, AQA
- Gases; measuring volumes, pages 140, 232, 237, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; natural gas, page 178, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; natural gas, pages 18, 21, GCSE Physics; The Revision Guide, CGP, AQA
- Gases; natural gas, pages 45, 46 54, 56, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Gases; natural gas, pages 47, 48, 56, 58, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Gases; particle model of, pages 330-1, GCSE Combined Science Trilogy 1, Hodder, AQA
- Gases; particle model of, pages 78-80, 85-6, GCSE Physics, Hodder, AQA
- Gases; particle motion, page 104, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Gases; particle motion, page 193, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; particle motion, pages 114, 115, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Gases; particle motion, pages 38, 41, GCSE Physics; The Revision Guide, CGP, AQA
- Gases; pressure, page 104, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Gases; pressure, page 36, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Gases; pressure, pages 114, 115, 169, 171, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Gases; states of matter, pages 106, 107, 110-112, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Gases; states of matter, pages 121, 122, 193, 195, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; states of matter, pages 38-40, GCSE Physics; The Revision Guide, CGP, AQA
- Gases; states of matter, pages 96, 97, 100-102, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Gases; tests for, page 153, GCSE Combined Science; The Revision Guide, CGP, AQA
- Gases; Volume and mass measurement, pages 120, GCSE Combined Science Trilogy 2, Hodder, AQA
- Gases; volume, pages 78-79, 129, GCSE Chemistry; Third Edition, Oxford University Press, AQA
Edexcel
- Gases, molar volumes of, pages 118-119, GCSE Chemistry, Pearson, Edexcel
- Gases, page 300, GCSE Physics, CGP, Edexcel
- Gases, pages 34, 35, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Gases, pages 96, 98, GCSE Chemistry, CGP, Edexcel
- Gases, pages 97, 98, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Gases; doing work, page 312, GCSE Physics, CGP, Edexcel
- Gases; pressure, page 424, GCSE Combined Science, Pearson Edexcel
- Gases; pressure, pages 192, 194-195, GCSE Physics, Pearson Edexcel
- Gases; pressure, pages 310-312, 319, GCSE Physics, CGP, Edexcel
- Gases; temperature, page 192, GCSE Physics, Pearson Edexcel
- Gases; temperature, page 424, GCSE Combined Science, Pearson Edexcel
- Gases; volume, pages 194-195, GCSE Physics, Pearson Edexcel
OCR
- Gases, page 12, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Gases, page 18, Gateway GCSE Chemistry, Oxford, OCR
- Gases, pages 14, 17, 18, Gateway GCSE Physics; The Revision Guide, CGP, OCR
- Gases, pages 82, 152, 155, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Gases; atmosphere, pages 252-257, Gateway GCSE Chemistry, Oxford, OCR
- Gases; calculating amounts, pages 170-171, Gateway GCSE Chemistry, Oxford, OCR
- Gases; changing state, pages 76-77, Gateway GCSE Chemistry, Oxford, OCR
- Gases; collecting, pages 128, 222, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Gases; collection, pages 265, 267, Gateway GCSE Chemistry, Oxford, OCR
- Gases; detection, pages 146-147, 265, 267, Gateway GCSE Chemistry, Oxford, OCR
- Gases; pressure in, pages 17, 18, Gateway GCSE Physics; The Revision Guide, CGP, OCR
- Gases; pressure, page 179, Gateway GCSE Chemistry, Oxford, OCR
- Gases; refined crude oil, page 239, Gateway GCSE Chemistry, Oxford, OCR
- Gases; volume, pages 170-171, 174, Gateway GCSE Chemistry, Oxford, OCR