Difference between revisions of "Hydrogen"
| Line 4: | Line 4: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
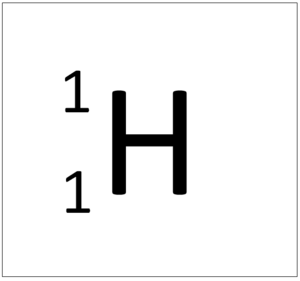
| + | [[File:HydrogenSymbol.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[hydrogen]].]] | ||
| + | |||
[[Hydrogen]] is a [[non-metal]] [[element]] with 1 [[proton]] in the [[Atomic Nucleus|nucleus]]. | [[Hydrogen]] is a [[non-metal]] [[element]] with 1 [[proton]] in the [[Atomic Nucleus|nucleus]]. | ||
===About Hydrogen=== | ===About Hydrogen=== | ||
| Line 9: | Line 11: | ||
: [[Hydrogen]] is a [[gas]] at [[STP|room temperature]]. | : [[Hydrogen]] is a [[gas]] at [[STP|room temperature]]. | ||
: [[Hydrogen]] [[gas]] is less [[density|dense]] than [[air]]. | : [[Hydrogen]] [[gas]] is less [[density|dense]] than [[air]]. | ||
| + | : An [[atom]] of [[hydrogen]] has one [[electron]]. | ||
| + | : A [[Hydrogen]] [[ion]] has usually lost its only [[electron]] to become [[Positive Charge|positively charged]]. | ||
===Testing for Hydrogen=== | ===Testing for Hydrogen=== | ||
#Collect the [[gas]] in a [[Test Tube|test tube]]. | #Collect the [[gas]] in a [[Test Tube|test tube]]. | ||
| Line 20: | Line 24: | ||
: [[Hydrogen]] is a [[gas]] at [[STP|standard temperature and pressure]]. | : [[Hydrogen]] is a [[gas]] at [[STP|standard temperature and pressure]]. | ||
: [[Hydrogen]] [[gas]] is less [[density|dense]] than [[air]]. | : [[Hydrogen]] [[gas]] is less [[density|dense]] than [[air]]. | ||
| + | : An [[atom]] of [[hydrogen]] has one [[electron]]. | ||
| + | : A [[Hydrogen]] [[ion]] has usually lost its only [[electron]] to become [[Positive Charge|positively charged]]. | ||
===Isotopes=== | ===Isotopes=== | ||
| − | : [[Hydrogen]] | + | {| class="wikitable" |
| − | : | + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] |
| − | : | + | | style="height:20px; width:200px; text-align:center;" |[[Deuterium]] |
| − | : | + | | style="height:20px; width:200px; text-align:center;" |[[Tritium]] |
| + | |- | ||
| + | |[[File:Hydrogen.png|center|200px]] | ||
| + | |[[File:Deuterium.png|center|200px]] | ||
| + | |[[File:Tritium.png|center|200px]] | ||
| + | |- | ||
| + | |[[File:HydrogenSymbol.png|center|200px]] | ||
| + | |[[File:DeuteriumSymbol.png|center|200px]] | ||
| + | |[[File:TritiumSymbol.png|center|200px]] | ||
| + | |- | ||
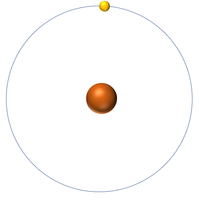
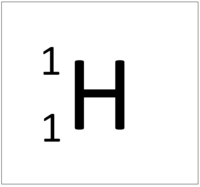
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] always has 1 [[proton]] but [[isotope]] there are no [[neutron]]s. | ||
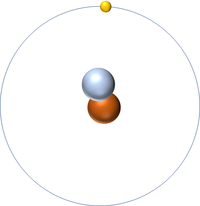
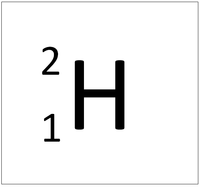
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] always has 1 [[proton]] but in this [[isotope]] there is 1 [[neutron]]. This [[isotope]] of [[Hydrogen]] is known as [[Deuterium]]. | ||
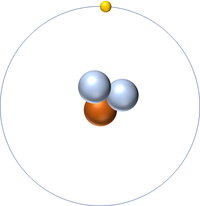
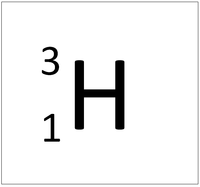
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] always has 1 [[proton]] but in this [[isotope]] there is 2 [[neutron]]s. This [[isotope]] of [[Hydrogen]] is known as [[Tritium]]. | ||
| + | |} | ||
===Testing for Hydrogen=== | ===Testing for Hydrogen=== | ||
#Collect the [[gas]] in a [[Test Tube|test tube]]. | #Collect the [[gas]] in a [[Test Tube|test tube]]. | ||
#Place a lit [[Wooden Splint|splint]] over the mouth of the [[Test Tube|test tube]]. | #Place a lit [[Wooden Splint|splint]] over the mouth of the [[Test Tube|test tube]]. | ||
#If a 'squeaky pop' [[sound]] is made then the [[gas]] is [[Hydrogen]]. | #If a 'squeaky pop' [[sound]] is made then the [[gas]] is [[Hydrogen]]. | ||
Revision as of 11:45, 31 March 2019
Contents
Key Stage 2
Meaning
Hydrogen is a gas that can catch fire easily.
Key Stage 3
Meaning
Hydrogen is a non-metal element with 1 proton in the nucleus.
About Hydrogen
- Hydrogen has the chemical formula H2.
- Hydrogen is a gas at room temperature.
- Hydrogen gas is less dense than air.
- An atom of hydrogen has one electron.
- A Hydrogen ion has usually lost its only electron to become positively charged.
Testing for Hydrogen
- Collect the gas in a test tube.
- Place a lit splint over the mouth of the test tube.
- If a 'squeaky pop' sound is made then the gas is Hydrogen.
Key Stage 4
Meaning
Hydrogen is a non-metal element with 1 proton in the nucleus.
About Hydrogen
- Hydrogen has the chemical formula H2.
- Hydrogen is a gas at standard temperature and pressure.
- Hydrogen gas is less dense than air.
- An atom of hydrogen has one electron.
- A Hydrogen ion has usually lost its only electron to become positively charged.
Isotopes
| Hydrogen | Deuterium | Tritium |
| Hydrogen always has 1 proton but isotope there are no neutrons. | Hydrogen always has 1 proton but in this isotope there is 1 neutron. This isotope of Hydrogen is known as Deuterium. | Hydrogen always has 1 proton but in this isotope there is 2 neutrons. This isotope of Hydrogen is known as Tritium. |