Difference between revisions of "Metal"
(→About Metals) |
|||
| Line 37: | Line 37: | ||
*[[Ductility|Ductile]] - They can be stretched into wires. | *[[Ductility|Ductile]] - They can be stretched into wires. | ||
*[[Sonorousness|Sonorous]] - They make a ringing sound when they are hit. | *[[Sonorousness|Sonorous]] - They make a ringing sound when they are hit. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | A [[Metal]] is a a [[material]] that is a good [[Electrical Conductor|conductor]] of [[electricity]] and a good [[Thermal Conductor|conductor]] of [[thermal energy]]. | ||
| + | |||
| + | ===About Metals=== | ||
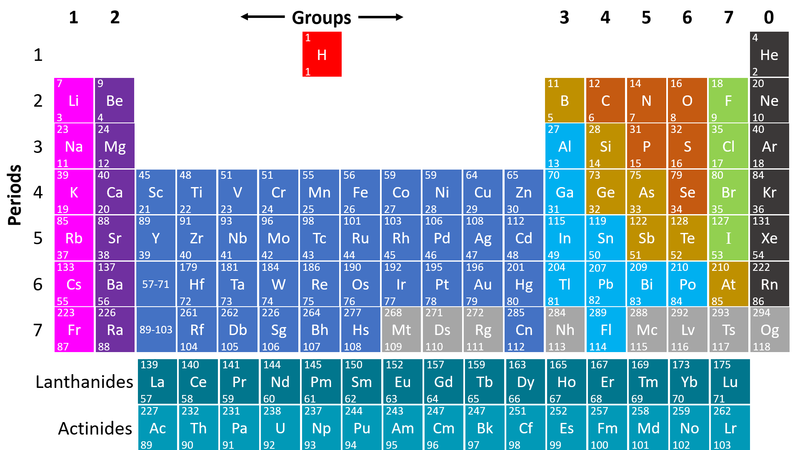
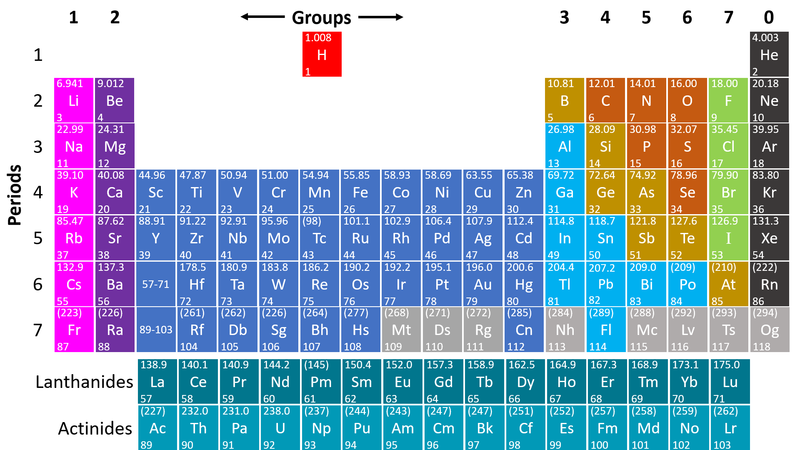
| + | : [[Metal]]s are found on the left hand side of the [[Periodic Table]] | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:PeriodicTableKS4.png|center|800px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |This [[Periodic Table]] shows the [[metal]] [[element]]s in blue and purple. | ||
| + | |} | ||
| + | |||
| + | : [[Metal]]s lose [[electron]]s to form [[Positive Ion|positive ions]] in [[compound]]s with [[non-metal]]s. | ||
| + | : [[Metal]]s form [[Hydroxide]]s when they [[Chemical Reaction|react]] with [[water]]. | ||
| + | : [[Metal]] [[element]]s form [[Metallic Bond|metallic bonds]] in which [[Positive Ion|positive ions]] are surrounded by a sea of [[Delocalised Electron|delocalised electrons]]. | ||
| + | : A [[metal]] made of more than one [[metal]] [[element]] is called an [[alloy]]. | ||
| + | : [[Metal]]s make good [[Electrical Conductor|electrical]] and [[Thermal Conductor|thermal]] [[conductor]]s because the [[Delocalised Electron|delocalised electrons]] are free to move around the [[material]]. | ||
Revision as of 00:29, 9 December 2018
Contents
Key Stage 1
Meaning
Metal is a hard, smooth, shiny, bendy and opaque material.
About Metals
There are many different types of metal but they are all similar in their properties. Metals are used to make knives and forks because they are hard and smooth. Metals are used to make wires because they are bendy. Metal can be used to make hammers because it is hard and strong.
Key Stage 3
Meaning
A Metal is a a material that is a good conductor of electricity and a good conductor of thermal energy.
About Metals
- Metals are found on the left hand side of the Periodic Table
| This Periodic Table shows the metal elements in blue and purple. |
Properties of Metals
There are several key properties of metals you should know. Metals are:
- Good conductors of electricity
- Good conductors of thermal energy
- Shiny - They have reflective surfaces when polished.
- Malleable - They can be hammered into shape.
- Ductile - They can be stretched into wires.
- Sonorous - They make a ringing sound when they are hit.
Key Stage 4
Meaning
A Metal is a a material that is a good conductor of electricity and a good conductor of thermal energy.
About Metals
- Metals are found on the left hand side of the Periodic Table
| This Periodic Table shows the metal elements in blue and purple. |
- Metals lose electrons to form positive ions in compounds with non-metals.
- Metals form Hydroxides when they react with water.
- Metal elements form metallic bonds in which positive ions are surrounded by a sea of delocalised electrons.
- A metal made of more than one metal element is called an alloy.
- Metals make good electrical and thermal conductors because the delocalised electrons are free to move around the material.