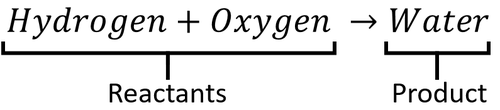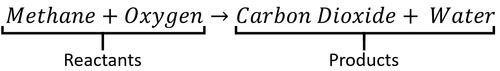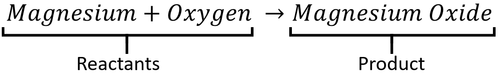Difference between revisions of "Word Equation"
(→About Word Equations) |
(→About Word Equations) |
||
| Line 29: | Line 29: | ||
===About Word Equations=== | ===About Word Equations=== | ||
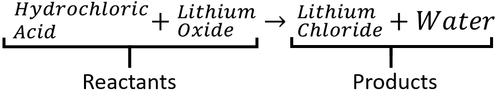
: In a word equation the [[reactant]]s are on the left and the [[product]]s are on the right. | : In a word equation the [[reactant]]s are on the left and the [[product]]s are on the right. | ||
| − | |||
| − | |||
===Examples=== | ===Examples=== | ||
Revision as of 21:48, 6 December 2018
Contents
Key Stage 3
Meaning
A word Equation is a way of representing chemical reactions to show the reactants and products using the names of the chemicals.
About Word Equations
- Word equations can be used to show the names of chemicals before and after reactions but they do not show the numbers of molecules or the number of atoms in each molecule. For that information you need a Balanced Symbol Equation.
Examples
Key Stage 4
Meaning
A word equation is a way of representing chemical reactions to show the reactants and products using the names of the chemicals.
About Word Equations
Examples
\({\frac{Calcium}{Carbonate}} \to {\tfrac{Calcium}{Oxide}} + {\tfrac{Carbon}{Dioxide}}\)
\(Calcium<sup>2</sup>Carbonate \to Calcium Oxide + Carbon dioxide\)