Electronic Structure
Contents
Key Stage 4
Meaning
The electronic configuration of an atom is the way the electrons are arranged in orbitals around the nucleus.
About The Electronic Configuration
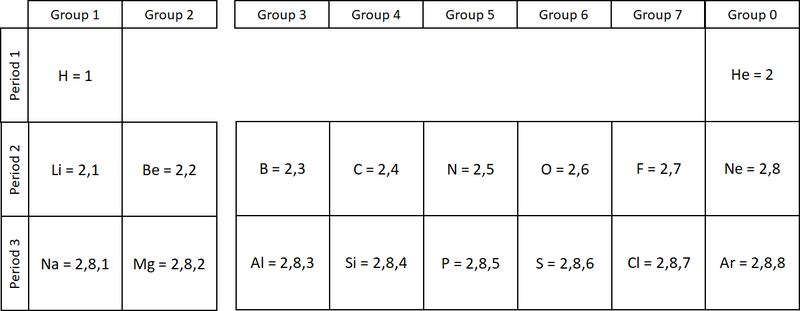
- The electronic configuration of an atom is written by stating the number of electrons in each electron shell.
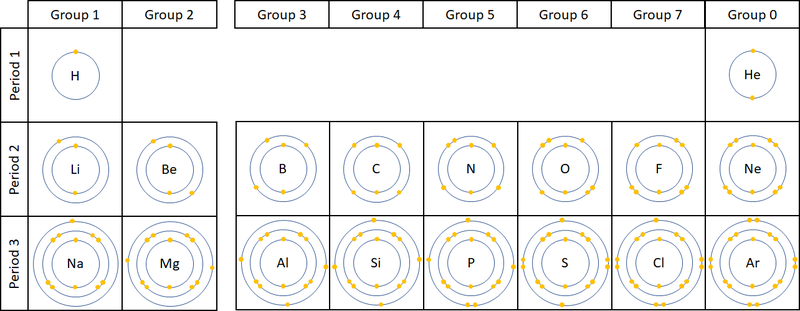
- As an example Argon has 2 electrons in the first shell, 8 in the second and 8 in the last, so the electronic configuration is 2,8,8.
| The two diagrams show how electrons are configured in the first 20 elements on the Periodic Table. |
References
AQA
- Electronic structure, page 121, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electronic structure, pages 28-31, 164, GCSE Chemistry; Student Book, Collins, AQA
- Electronic structure; and the periodic table, pages 127-8, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electronic structure; stable, pages 38, 43, 59, 60-1, 164-5, GCSE Chemistry; Student Book, Collins, AQA
- Electronic structures, pages 12-14, 18-19, 24-25, 30-31, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Electronic structures, pages 20, 23, 28, GCSE Chemistry; The Revision Guide, CGP, AQA
- Electronic structures, pages 44, 45, 71, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Electronic structures, pages 44, 45, 73, GCSE Chemistry, CGP, AQA