Difference between revisions of "Radon"
| Line 21: | Line 21: | ||
: [[Radon]] is a [[gas]] at [[STP|standard temperature and pressure]]. | : [[Radon]] is a [[gas]] at [[STP|standard temperature and pressure]]. | ||
: An [[atom]] of [[Radon]] has a full [[Outer Shell|outer shell]] so it is [[inert]]. | : An [[atom]] of [[Radon]] has a full [[Outer Shell|outer shell]] so it is [[inert]]. | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Radon gas, page 97, GCSE Physics, Hodder, AQA ''] | ||
Revision as of 23:31, 10 November 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
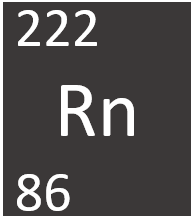
Radon is a radioactive Group 0 element, on the Periodic Table, with an atomic number of 86.
About Radon
- Radon has the chemical formula Rn.
- Radon has 86 protons and 136 neutrons in its nucleus giving it an Atomic Number of 86 and an atomic mass of 222.
- Radon is a Noble Gas.
- Radon is a gas at room temperature.
- An atom of Radon has a full outer shell so it is inert.
Key Stage 4
Meaning
Radon is a radioactive Group 0 element, on the Periodic Table, with 86 protons in the nucleus.
About Radon
- Radon has the chemical formula Rn.
- The most stable isotope of Radon has 136 neutrons in its nucleus giving it an atomic mass of 222.
- Radon is a Noble Gas.
- Radon is a gas at standard temperature and pressure.
- An atom of Radon has a full outer shell so it is inert.