Difference between revisions of "Atom"
| Line 5: | Line 5: | ||
===About Atoms in The Dalton Model=== | ===About Atoms in The Dalton Model=== | ||
: In [[The Dalton Model]] [[atom]]s are shown as [[sphere|ball]] shaped [[particle]]s. This makes it easier to draw [[diagram]]s of [[molecule]]s. | : In [[The Dalton Model]] [[atom]]s are shown as [[sphere|ball]] shaped [[particle]]s. This makes it easier to draw [[diagram]]s of [[molecule]]s. | ||
| − | + | {| class="wikitable" | |
| + | |- | ||
| + | |[[File:DaltonModelAtom.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A picture of [[The Dalton Model]] of an [[atom]]. | ||
| + | |} | ||
===About Atoms beyond The Dalton Model=== | ===About Atoms beyond The Dalton Model=== | ||
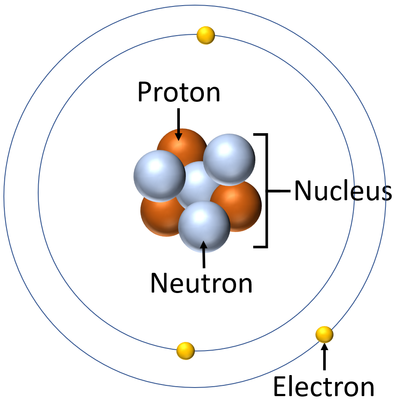
: [[Atom]]s are made of three smaller [[particle]]s; the [[proton]], [[neutron]] and [[electron]]. | : [[Atom]]s are made of three smaller [[particle]]s; the [[proton]], [[neutron]] and [[electron]]. | ||
: [[Proton]]s and [[neutron]]s are found in the [[nucleus]] at the centre of an [[atom]]. [[Electron]]s are found [[orbiting]] the [[nucleus]] in 'shells'. | : [[Proton]]s and [[neutron]]s are found in the [[nucleus]] at the centre of an [[atom]]. [[Electron]]s are found [[orbiting]] the [[nucleus]] in 'shells'. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:AtomDiagram.png|center|400px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of an [[atom]]. | ||
| + | |} | ||
| + | |||
: In an [[atom]] the number of [[proton]]s is always the same as the number of [[electron]]s. | : In an [[atom]] the number of [[proton]]s is always the same as the number of [[electron]]s. | ||
: Different [[atom]]s can have different numbers of [[proton]]s and [[neutron]]s. | : Different [[atom]]s can have different numbers of [[proton]]s and [[neutron]]s. | ||
: The simplest [[atom]] is [[Hydrogen]] which has 1 [[proton]] and 1 [[electron]] and no [[neutron]]s. | : The simplest [[atom]] is [[Hydrogen]] which has 1 [[proton]] and 1 [[electron]] and no [[neutron]]s. | ||
Revision as of 11:58, 23 September 2018
Contents
Key Stage 3
Meaning
An atom is a very small particle made of protons, neutrons and electrons that can join with other atoms to make molecules.
About Atoms in The Dalton Model
- In The Dalton Model atoms are shown as ball shaped particles. This makes it easier to draw diagrams of molecules.
| A picture of The Dalton Model of an atom. |
About Atoms beyond The Dalton Model
- Atoms are made of three smaller particles; the proton, neutron and electron.
- Protons and neutrons are found in the nucleus at the centre of an atom. Electrons are found orbiting the nucleus in 'shells'.
| A diagram of an atom. |