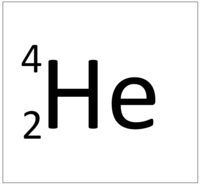Difference between revisions of "Atomic Number"
| Line 41: | Line 41: | ||
: [[Proton]]s have a [[Relative Atomic Charge|relative atomic charge]] of +1 so the number of [[proton]]s determines the [[Relative Atomic Charge|relative atomic charge]] of the [[Atomic Nucleus|atomic nucleus]]. | : [[Proton]]s have a [[Relative Atomic Charge|relative atomic charge]] of +1 so the number of [[proton]]s determines the [[Relative Atomic Charge|relative atomic charge]] of the [[Atomic Nucleus|atomic nucleus]]. | ||
: The number of [[electron]]s [[orbit]]ing the [[Atomic Nucleus|nucleus]] is the same as the number of [[proton]]s in the [[Atomic Nucleus|nucleus]] of an [[atom]]. | : The number of [[electron]]s [[orbit]]ing the [[Atomic Nucleus|nucleus]] is the same as the number of [[proton]]s in the [[Atomic Nucleus|nucleus]] of an [[atom]]. | ||
| + | |||
| + | ===Examples==={| class="wikitable" | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Helium''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Lithium''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Beryllium''' | ||
| + | |- | ||
| + | |[[File:Hydrogen.png|center|200px]] | ||
| + | |[[File:Helium.png|center|200px]] | ||
| + | |[[File:Lithium.png|center|200px]] | ||
| + | |[[File:Beryllium.png|center|200px]] | ||
| + | |- | ||
| + | |[[File:HydrogenSymbol.png|center|200px]] | ||
| + | |[[File:HeliumSymbol.png|center|200px]] | ||
| + | |[[File:LithiumSymbol.png|center|200px]] | ||
| + | |[[File:BerylliumSymbol.png|center|200px]] | ||
| + | |- | ||
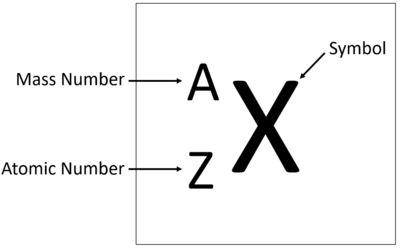
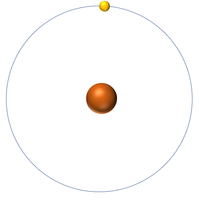
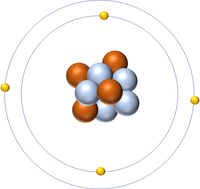
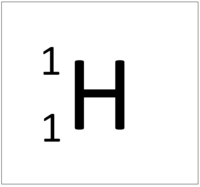
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] has 1 [[proton]] so its '''atomic number''' is 1 and the [[Relative Atomic Charge|relative atomic charge]] of the [[Atomic Nucleus|nucleus]] is +1. | ||
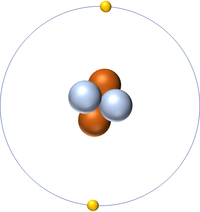
| + | | style="height:20px; width:200px; text-align:center;" |[[Helium]] has 2 [[proton]]s so its '''atomic number''' is 2 and the [[Relative Atomic Charge|relative atomic charge]] of the [[Atomic Nucleus|nucleus]] is +2. | ||
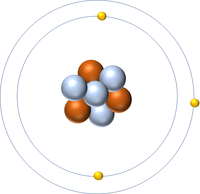
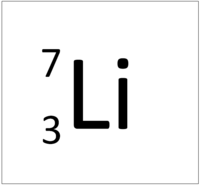
| + | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] has 3 [[proton]]s so its '''atomic number''' is 3 and the [[Relative Atomic Charge|relative atomic charge]] of the [[Atomic Nucleus|nucleus]] is +3. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Beryllium]] has 4 [[proton]]s so its '''atomic number''' is 4 and the [[Relative Atomic Charge|relative atomic charge]] of the [[Atomic Nucleus|nucleus]] is +4. | ||
| + | |} | ||
Revision as of 19:01, 25 November 2018
Contents
Key Stage 3
Meaning
The Atomic Number is the number of protons in the nucleus of an atom.
About The Atomic Number
- The Atomic Number of an atom determines which element it is.
- The number of protons also determines the number of electrons.
Examples
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has 1 proton so its atomic number is 1. | Helium has 2 protons so its atomic number is 2. | Lithium has 3 protons so its atomic number is 3. | Beryllium has 4 protons so its atomic number is 4. |
Key Stage 4
Meaning
The Atomic Number is the number of protons in the nucleus of an atom.
About The Atomic Number
- The Atomic Number of an atom determines which element it is.
- Protons have a relative atomic charge of +1 so the number of protons determines the relative atomic charge of the atomic nucleus.
- The number of electrons orbiting the nucleus is the same as the number of protons in the nucleus of an atom.
===Examples==={| class="wikitable" | style="height:20px; width:200px; text-align:center;" |Hydrogen | style="height:20px; width:200px; text-align:center;" |Helium | style="height:20px; width:200px; text-align:center;" |Lithium | style="height:20px; width:200px; text-align:center;" |Beryllium |-
|
|
|
|
|-
|
|
|
|
|- | style="height:20px; width:200px; text-align:center;" |Hydrogen has 1 proton so its atomic number is 1 and the relative atomic charge of the nucleus is +1. | style="height:20px; width:200px; text-align:center;" |Helium has 2 protons so its atomic number is 2 and the relative atomic charge of the nucleus is +2. | style="height:20px; width:200px; text-align:center;" |Lithium has 3 protons so its atomic number is 3 and the relative atomic charge of the nucleus is +3. | style="height:20px; width:200px; text-align:center;" |Beryllium has 4 protons so its atomic number is 4 and the relative atomic charge of the nucleus is +4. |}