Difference between revisions of "Separating Mixtures"
(→About Separating Mixtures) |
|||
| Line 72: | Line 72: | ||
Different '''separation''' techniques are needed for different types of [[mixture]]: | Different '''separation''' techniques are needed for different types of [[mixture]]: | ||
*[[Filtration]] - To remove an [[insoluble]] [[solid]] from [[mixture]] with a [[liquid]]. | *[[Filtration]] - To remove an [[insoluble]] [[solid]] from [[mixture]] with a [[liquid]]. | ||
| − | *[[Evaporation of Solutions|Evaporation]] ( | + | *[[Evaporation of Solutions|Evaporation]] ([[Evaporation of Solutions|Crystallisation]]) - Collecting a [[solute]] from a [[solution]] while losing the [[solvent]]. |
*[[Distillation]] - Collecting both the [[solute]] and [[solvent]] from a [[solution]]. | *[[Distillation]] - Collecting both the [[solute]] and [[solvent]] from a [[solution]]. | ||
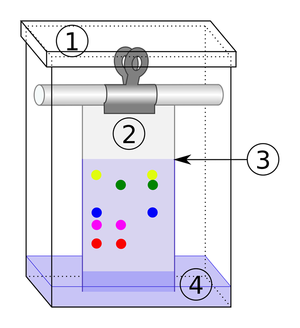
*[[Chromatography]] - '''Separating''' two or more [[solute]]s in [[solution]]. | *[[Chromatography]] - '''Separating''' two or more [[solute]]s in [[solution]]. | ||
Revision as of 14:44, 20 January 2019
Contents
Key Stage 2
Meaning
Separating Mixtures is when you take out one of the substances from a mixture.
About Separating Mixtures
- Different mixtures need to be separated in different ways:
- Sieving
- Filtering
- Evaporating
Examples
| Beach | Muddy Puddle | Sea Water |
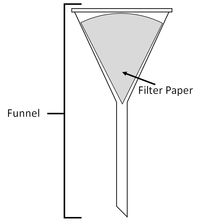
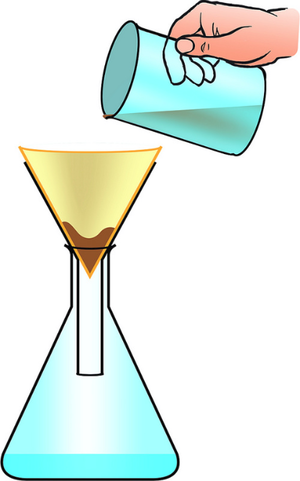
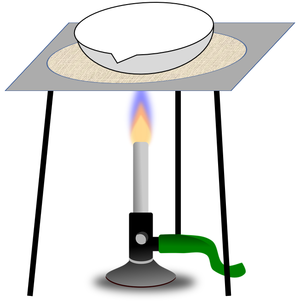
| You can separate the sand from the pebbles with a sieve. | You can separate mud from water in the puddle with filter paper and a funnel. | You can separate salt from the water by evaporating the water in an evaporating dish. |
Key Stage 3
Meaning
Separating Mixtures is when you take out one or more of the substances from a mixture.
About Separating Mixtures
- All mixtures can be separated without a chemical reaction.
- There are several methods of separating mixtures that you should know:
Examples
| Filtration | Evaporation |
| A mixture or a liquid and an insoluble solid can be separated by filtration. | A solute can be recovered from a solution by evaporating away the solvent. |
| Distillation | Chromatography |
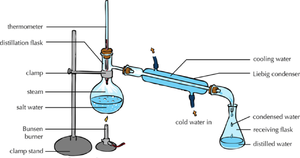
| The solute and solvent can be separated by distillation. | When there are more than 1 solutes in a solution chromatography can separate them. |
Key Stage 4
Meaning
To separate a mixture is to remove one or more chemicals from a mixture of chemicals.
About Separating Mixtures
Different separation techniques are needed for different types of mixture:
- Filtration - To remove an insoluble solid from mixture with a liquid.
- Evaporation (Crystallisation) - Collecting a solute from a solution while losing the solvent.
- Distillation - Collecting both the solute and solvent from a solution.
- Chromatography - Separating two or more solutes in solution.