Difference between revisions of "Covalent Bond"
| Line 39: | Line 39: | ||
:[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Covalent bonds; giant molecular structures, page 45, GCSE Chemistry, Pearson, Edexcel ''] | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Covalent bonds; giant molecular structures, page 45, GCSE Chemistry, Pearson, Edexcel ''] | ||
:[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Covalent bonds; simple molecular structures, page 42, GCSE Chemistry, Pearson, Edexcel ''] | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Covalent bonds; simple molecular structures, page 42, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Covalent bonds, pages 60-65, 74-75, 77, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 01:06, 4 December 2019
Contents
Key Stage 4
Meaning
A covalent bond is a type of chemical bond in which atoms share electrons with one another.
About Covalent Bonds
- Atoms are more chemically stable when their outer shell is full of electrons. One way atoms can have a full outer shell is by sharing some electrons with other atoms, this is a covalent bond.
- Covalent bonds happen between non-metal elements.
- Covalent bonds can be represented by a Dot and Cross Diagram to show how the electrons are shared between the outer shells of different atoms.
Examples
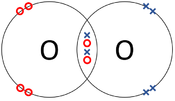
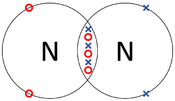
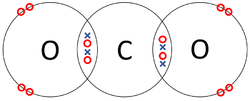
| The two Oxygen atoms each share two of their electrons with one another. | The two Nitrogen atoms each share three of their electrons with one another. | Each Oxygen shares two of its electrons with the Carbon atom while the Carbon atom shares two electrons with each Oxygen atom. |
References
AQA
- Covalent bond, pages 57-9, 62-3, 72-5, GCSE Chemistry; Student Book, Collins, AQA
- Covalent bonds, page 116, GCSE Combined Science; The Revision Guide, CGP, AQA
- Covalent bonds, page 31, GCSE Chemistry; The Revision Guide, CGP, AQA
- Covalent bonds, pages 154, 156-7, 163, GCSE Combined Science Trilogy 1, Hodder, AQA
- Covalent bonds, pages 29, 78-88, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Covalent bonds, pages 29, 80-90, GCSE Chemistry, CGP, AQA
- Covalent bonds, pages 38, 44-51, 149, 224-225, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Covalent bonds, pages 41-2, 47-8, GCSE Chemistry, Hodder, AQA
- Covalent bonds; giant covalent substances, page 44, GCSE Chemistry, Hodder, AQA
- Covalent bonds; giant covalent substances, pages 159-60, GCSE Combined Science Trilogy 1, Hodder, AQA
Edexcel
- Covalent bonds, pages 184-185, 186, 261, GCSE Combined Science, Pearson Edexcel
- Covalent bonds, pages 40-41, 42, 147, GCSE Chemistry, Pearson, Edexcel
- Covalent bonds; giant molecular structures, page 45, GCSE Chemistry, Pearson, Edexcel
- Covalent bonds; simple molecular structures, page 42, GCSE Chemistry, Pearson, Edexcel