Difference between revisions of "Separating Mixtures"
(→Examples) |
(→Examples) |
||
| Line 43: | Line 43: | ||
===Examples=== | ===Examples=== | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Filtration''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Evaporation''' | ||
|- | |- | ||
|[[File:Filtration.png|center|300px]] | |[[File:Filtration.png|center|300px]] | ||
|[[File:EvaporatingClipart.png|center|300px]] | |[[File:EvaporatingClipart.png|center|300px]] | ||
|- | |- | ||
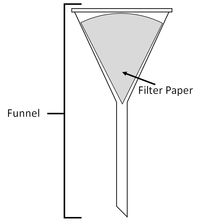
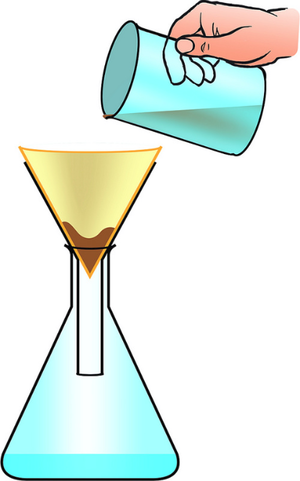
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |A [[mixture]] or a [[liquid]] and an [[insoluble]] [[solid]] can be '''separated''' by [[filtration]]. |
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |A [[solute]] can be recovered from a [[solution]] by [[evaporating]] away the [[solvent]]. |
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Distillation''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Chromatography''' | ||
|- | |- | ||
|[[File:Distillation.png|center|300px]] | |[[File:Distillation.png|center|300px]] | ||
|[[File:ChromatographyDiagram.png|center|300px]] | |[[File:ChromatographyDiagram.png|center|300px]] | ||
|- | |- | ||
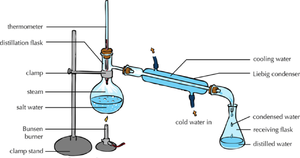
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |The [[solute]] and [[solvent]] can be '''separated''' by [[distillation]]. |
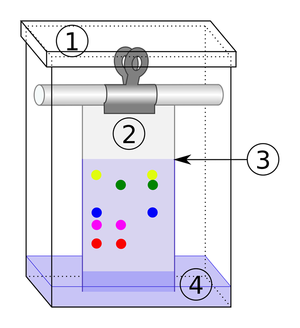
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |When there are more than 1 [[solute]]s in a [[solution]] [[chromatography]] can '''separate''' them. |
|} | |} | ||
Revision as of 11:08, 27 September 2018
Contents
Key Stage 2
Meaning
Separating Mixtures is when you take out one of the substances from a mixture.
About Separating Mixtures
- Different mixtures need to be separated in different ways:
- Sieving
- Filtering
- Evaporating
Examples
| Beach | Muddy Puddle | Sea Water |
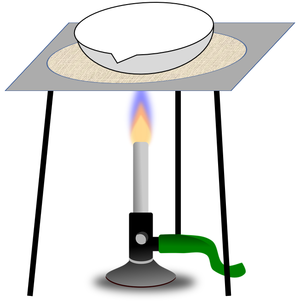
| You can separate the sand from the pebbles with a sieve. | You can separate mud from water in the puddle with filter paper and a funnel. | You can separate salt from the water by evaporating the water in an evaporating dish. |
Key Stage 3
Meaning
Separating Mixtures is when you take out one or more of the substances from a mixture.
About Separating Mixtures
- All mixtures can be separated without a chemical reaction.
- There are several methods of separating mixtures that you should know:
Examples
| Filtration | Evaporation |
| A mixture or a liquid and an insoluble solid can be separated by filtration. | A solute can be recovered from a solution by evaporating away the solvent. |
| Distillation | Chromatography |
| The solute and solvent can be separated by distillation. | When there are more than 1 solutes in a solution chromatography can separate them. |