Difference between revisions of "Radon"
| Line 4: | Line 4: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
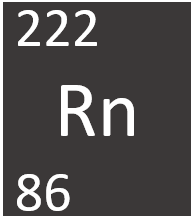
| − | [[File: | + | [[File:RadonSymbol1.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Radon]].]] |
[[Radon]] is a [[radioactive]] [[Group 0]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 86. | [[Radon]] is a [[radioactive]] [[Group 0]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 86. | ||
===About Radon=== | ===About Radon=== | ||
Revision as of 21:22, 2 April 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Radon is a radioactive Group 0 element, on the Periodic Table, with an atomic number of 86.
About Radon
- Radon has the chemical formula Rn.
- Radon has 86 protons and 136 neutrons in its nucleus giving it an Atomic Number of 86 and an atomic mass of 222.
- Radon is a Noble Gas.
- Radon is a gas at room temperature.
- An atom of Radon has a full outer shell so it is inert.
Key Stage 4
Meaning
Radon is a radioactive Group 0 element, on the Periodic Table, with 86 protons in the nucleus.
About Radon
- Radon has the chemical formula Rn.
- The most stable isotope of Radon has 136 neutrons in its nucleus giving it an atomic mass of 222.
- Radon is a Noble Gas.
- Radon is a gas at standard temperature and pressure.
- An atom of Radon has a full outer shell so it is inert.