Difference between revisions of "Reactivity"
| Line 39: | Line 39: | ||
====Reactivity along Period 2==== | ====Reactivity along Period 2==== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:Period2ElectronShells.png|center|600px]] | ||
| + | |- | ||
| + | |[[File:Period2ElectronShielding.png|center|600px]] | ||
| + | |- | ||
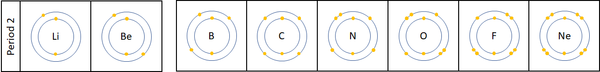
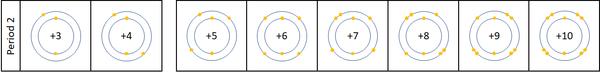
| + | | style="height:20px; width:600px; text-align:left;" |For the first 3 [[element]]s [[Lithium]], [[Beryllium]] and [[Boron]] all lose [[electron]]s in [[Chemical Reaction|chemical reactions]]. | ||
| + | |||
| + | The [[reactivity]] decreases as you go across the [[Period (Chemistry)|period]]: | ||
| + | *The outer [[electron]]s all roughly the same distance away from the [[Atomic Nucleus|nucleus]] | ||
| + | *The [[charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]]. | ||
| + | |||
| + | |} | ||
Revision as of 16:43, 5 December 2018
Contents
Key Stage 4
Meaning
Reactivity is how vigorously a chemical will react.
About Reactivity
- Reactivity is determined by how easily an element can lose or gain electrons.
- Electrons are held in orbit around the nucleus because the electrons are negatively charged and are attracted to the nucleus which is positively charged.
- If an element loses electrons easily it is highly reactive.
- If an element gains electrons readily it is also highly reactive.
Three important factors affect reactivity of elements.
- The charge of the nucleus
- The shielding effect of inner electrons.
- Distance between the nucleus and the outer shell.
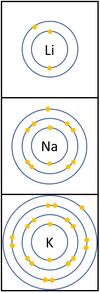
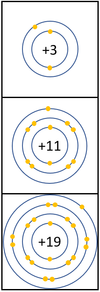
Reactivity in Groups 1, 2 and 3
| In a chemical reaction the electron in the outer shell is lost.
The reactivity increases as you go down the group because:
|
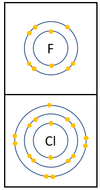
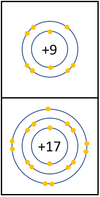
Reactivity in Group 7
| In a chemical reaction an extra electron is added to the outer shell.
The reactivity decreases as you go down the group because:
|
Reactivity along Period 2
| For the first 3 elements Lithium, Beryllium and Boron all lose electrons in chemical reactions.
The reactivity decreases as you go across the period:
|