Boron
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
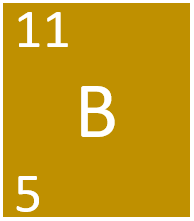
Boron is a Group 3 metalloid element, on the Periodic Table, with an atomic number of 5.
About Boron
- Boron has the chemical symbol B.
Atomic Structure
- Boron as 5 protons and 6 neutrons in its nucleus giving it an Atomic Number of 5 and an atomic mass of 11.
- Boron is in Period 2 of the Periodic Table because it has 2 electron shells.
Properties
- Boron is a shiny solid at room temperature.
Key Stage 4
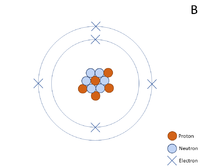
A 2 dimensional representation of the Bohr Model of a Boron-11 isotope with 5 protons and 6 neutrons in the nucleus and 2 electrons in the first shell and 3 in the outer shell.
Meaning
Boron is a Group 3 metalloid element, on the Periodic Table, with 5 protons in the nucleus.
About Boron
- Boron has the chemical symbol B.
Atomic Structure
- The most stable isotope of Boron has 6 neutrons in its nucleus giving it an atomic mass of 11.
- Boron is in Period 2 of the Periodic Table because it has 2 electron shells.
- Boron loses electrons to form positive metal ions.
Properties
- Boron is a metalloid element so in some conditions it is an electrical conductor.
- Boron is a shiny solid at standard temperature and pressure and has a high melting point.