Period (Chemistry)
Contents
Key Stage 3
Meaning
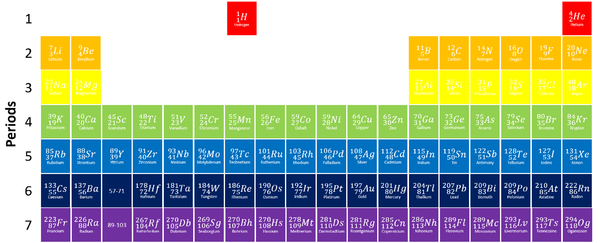
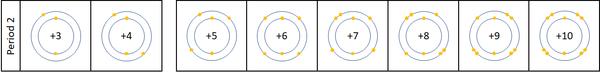
A period is a row on the Periodic Table whose elements all have the same number of Electron Shells.
About Periods
- Atomic Number increases as you move along a period.
Trends within Periods
- The chemical and physical properties of elements change as you move along a period.
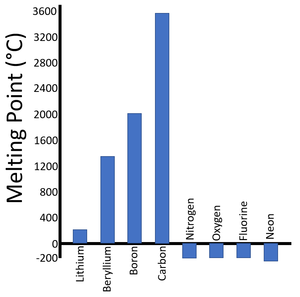
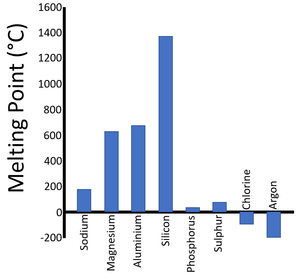
| Period 2 Melting Points | Period 3 Melting Points |
| There is a trend in the Melting Points as you move along the period. | A similar trend can be seen in the next period. |
Key Stage 4
Meaning
A period is a row on the Periodic Table whose elements all have the same number of Electron Shells.
About Periods
- Atomic Number increases as you move along a period.
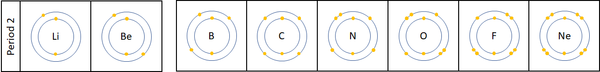
- The number of the period is the same as the number of electron shells.
Trends within Periods
| For the first 3 elements Lithium, Beryllium and Boron all lose electrons in chemical reactions.
The reactivity decreases as you go across the period because:
Nitrogen, Oxygen and Fluorine can all gain electrons to become negative ions in certain reactions. The reactivity increases as you go across the period because:
|