Contents
Key Stage 4
Meaning
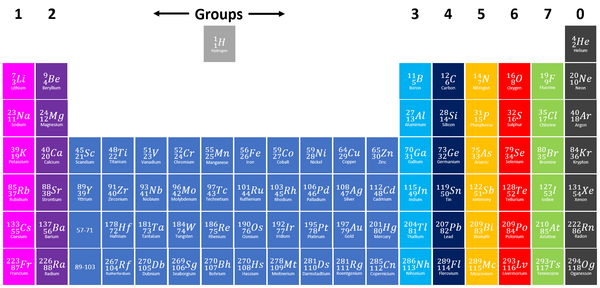
Group 0 elements, also known as Noble Gases on the Periodic Table are the elements which have a full outer shell.
| Group 0 elements are shown in grey at the right of the Periodic Table. |
About the Noble Gases
- Group 0 used to be called Group 8 but this caused confusion because most elements in Group 8 have 8 electrons in their Outer Shell but Helium only has 2, so it was renamed Group 0.
- The Noble Gases are all inert which means they do not react with other atoms.
- The Noble Gases are all monatomic which means they exist only as single atoms and do not join to form any molecules.
The Noble Gases in order of the number of electron shells:
Chemical Properties
- The Noble Gases are all inert (unreactive) because they have a full outer shell.
Physical Properties
- The Noble Gases are all gases at room temperature.
- The density and boiling point all increase as you go down the Periodic Table.
References
AQA
- Group 0 (noble gases), pages 128-9, GCSE Combined Science Trilogy 1, Hodder, AQA
- Group 0 (noble gases), pages 40-1, GCSE Chemistry; Student Book, Collins, AQA
- Group 0, page 111, GCSE Combined Science; The Revision Guide, CGP, AQA
- Group 0, page 26, GCSE Chemistry; The Revision Guide, CGP, AQA
- Group 0, pages 24-25, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Group 0, pages 64, 65, GCSE Chemistry, CGP, AQA
- Group 0, pages 64, 65, GCSE Combined Science Trilogy; Chemistry, CGP, AQA