Contents
Key Stage 4
Meaning
Fractional distillation is a technique which can be used to separate two or more solvents from solution.
About Fractional Distillation
Fractional Distillation can be used for:
- Separating a solution to recover multiple solvents.
Fractional Distillation cannot be used for:
- Separating two solutes from each other in solution - Chromatography
- Separating an insoluble solid from a soluble solid - Filtration
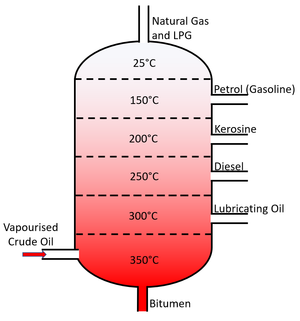
About the Fractional Distillation of Crude Oil
- Crude Oil is separated using fractional distillation.
- Fractional distillation is used to separate the different fractions of crude oil which each have different boiling points.
- Fractional Distillation relies on the different boiling points of liquids. By heating all liquids beyond their Boiling Point they can be turned into a gas. Each gas can then be cooled and condensed in tubes kept just below the boiling point of each fraction.
- The fractions of crude oil which can be separated during fractional distillation are:
References
AQA
- Fractional distillation of crude oil, pages 144-5, GCSE Combined Science Trilogy 2, Hodder, AQA
- Fractional distillation, page 18, 76, GCSE Chemistry; The Revision Guide, CGP, AQA
- Fractional distillation, page 24, GCSE Chemistry, Hodder, AQA
- Fractional distillation, pages 10, 150-151, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Fractional distillation, pages 137, 139, GCSE Combined Science Trilogy 1, Hodder, AQA
- Fractional distillation, pages 19, 230-1, 263, GCSE Chemistry; Student Book, Collins, AQA
- Fractional distillation, pages 40, 191, 192, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Fractional distillation, pages 40, 223, 224, GCSE Chemistry, CGP, AQA
- Fractional distillation; of crude oil, pages 173-4, GCSE Chemistry, Hodder, AQA