Difference between revisions of "Atom"
(→About Atoms beyond The Dalton Model) |
(→About Atoms beyond The Dalton Model) |
||
| Line 21: | Line 21: | ||
|} | |} | ||
| − | : In an [[atom]] the number of [[electron]]s is always the same as the number of [[ | + | : In an [[atom]] the number of [[electron]]s is always the same as the number of [[proton]]s in the [[nucleus]]. |
: Different [[atom]]s can have different numbers of [[proton]]s and [[neutron]]s. | : Different [[atom]]s can have different numbers of [[proton]]s and [[neutron]]s. | ||
: The simplest [[atom]] is [[Hydrogen]] which has 1 [[proton]] and 1 [[electron]] and no [[neutron]]s. | : The simplest [[atom]] is [[Hydrogen]] which has 1 [[proton]] and 1 [[electron]] and no [[neutron]]s. | ||
Revision as of 11:51, 23 November 2018
Contents
Key Stage 3
Meaning
An atom is a very small particle made of protons, neutrons and electrons that can join with other atoms to make molecules.
About Atoms in The Dalton Model
- In The Dalton Model atoms are shown as ball shaped particles. This makes it easier to draw diagrams of molecules.
| A picture of The Dalton Model of an atom. |
About Atoms beyond The Dalton Model
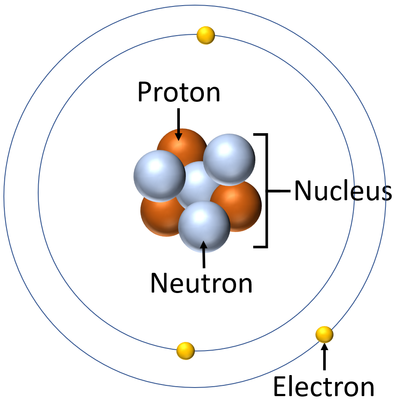
- Atoms are made of three smaller particles; the proton, neutron and electron.
- Protons and neutrons are found in the nucleus at the centre of an atom. Electrons are found orbiting the nucleus in 'shells'.
| A diagram of an atom. |