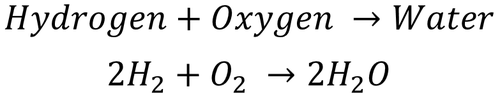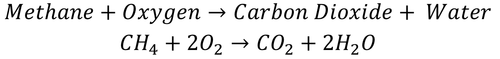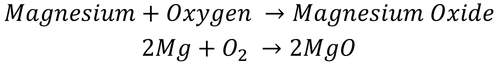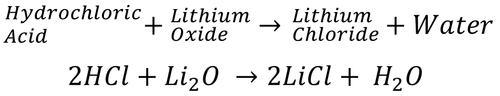Key Stage 3
Meaning
A Balanced Symbol Equation is a way to show the reactants and products in a chemical reaction using the chemical formulae of the reactants and products.
About Balanced Symbol Equations
- Balanced Symbol Equations shows the number of molecules of each chemical and the numbers of each atom within those chemicals.
- Balanced Symbol Equations can be used to find the quantity of each chemical needed to have a complete chemical reaction.
| This shows there are two H2 molecules and one O2 molecule needed to make two molecules of H2O. |
| This shows there is one CH4 molecule and two O2 molecules needed to make one molecule of CO2 and two molecules of H2O. |
| This shows there are two atoms of Mg and one molecule of O2 needed to make two molecules of MgO. |
| This shows there are two HCl molecules and one Li2O molecule needed to make two molecules of LiCl and one molecule of H2O. |
Balancing Equations
- You may know the chemical formulae of the reactants and products but not know the number of each chemical needed. For that you must balance the equation.