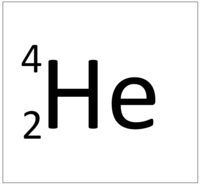Difference between revisions of "Helium"
(→About Helium) |
|||
| Line 18: | Line 18: | ||
===About Helium=== | ===About Helium=== | ||
: [[Helium]] has the [[Chemical Formula|chemical formula]] [[Helium|He]]. | : [[Helium]] has the [[Chemical Formula|chemical formula]] [[Helium|He]]. | ||
| − | : The most [[Stable Isotope|stable isotope]] of [[Helium]] has two [[neutron]]s in its [[Atomic Nucleus|nucleus]] an [[Relative Atomic Mass|atomic mass]] of 4. | + | : The most [[Stable Isotope|stable isotope]] of [[Helium]] has two [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 4. |
: [[Helium]] is a [[Noble Gas]]. | : [[Helium]] is a [[Noble Gas]]. | ||
: [[Helium]] is a [[gas]] at [[STP|standard temperature and pressure]]. | : [[Helium]] is a [[gas]] at [[STP|standard temperature and pressure]]. | ||
Revision as of 12:44, 31 March 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Helium is a Group 0 element an atomic number of 2.
About Helium
- Helium has the chemical formula He.
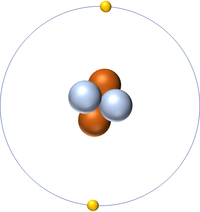
- Helium has two protons and two neutrons in its nucleus giving it an Atomic Number of 2 and an atomic mass of 4.
- Helium is a Noble Gas.
- Helium is a gas at room temperature.
- Helium gas is less dense than air.
- An atom of Helium has a full outer shell of two electrons so it is inert.
Key Stage 4
Meaning
Helium is a Group 0 element with 2 protons in the nucleus.
About Helium
- Helium has the chemical formula He.
- The most stable isotope of Helium has two neutrons in its nucleus giving it an atomic mass of 4.
- Helium is a Noble Gas.
- Helium is a gas at standard temperature and pressure.
- Helium gas is less dense than air.
- An atom of Helium has a full outer shell of two electrons so it is inert.
| Helium always has 2 protons. The most stable isotope has 2 neutrons. | |