Difference between revisions of "Ice-Water Anomaly"
(→Extra Information) |
|||
| Line 22: | Line 22: | ||
: [[Water]] is known as a [[Polar Molecule|polar molecule]] which means one part is slightly [[Positive Charge|positively charged]] and the other is slightly [[Negative Charge|negatively charged]]. Since there is strong [[force]] of [[attraction]] pulling the [[electron]]s towards to [[Oxygen]] [[atom]] in [[water]] then the [[Oxygen]] becomes slightly [[Negative Charge|negative]] and the [[Hydrogen]] [[atom]]s become slightly [[Positive Charge|positively charged]]. This causes the [[molecule]]s to line up with a [[Hydrogen]] of one [[molecule]] touching the [[Oxygen]] in another [[molecule]]. | : [[Water]] is known as a [[Polar Molecule|polar molecule]] which means one part is slightly [[Positive Charge|positively charged]] and the other is slightly [[Negative Charge|negatively charged]]. Since there is strong [[force]] of [[attraction]] pulling the [[electron]]s towards to [[Oxygen]] [[atom]] in [[water]] then the [[Oxygen]] becomes slightly [[Negative Charge|negative]] and the [[Hydrogen]] [[atom]]s become slightly [[Positive Charge|positively charged]]. This causes the [[molecule]]s to line up with a [[Hydrogen]] of one [[molecule]] touching the [[Oxygen]] in another [[molecule]]. | ||
| − | ==Extra Information== | + | ===Extra Information=== |
{{#ev:youtube|https://www.youtube.com/watch?v=UukRgqzk-KE}} | {{#ev:youtube|https://www.youtube.com/watch?v=UukRgqzk-KE}} | ||
Latest revision as of 18:39, 20 April 2019
Contents
Key Stage 3
Meaning
The Ice-Water Anomaly is the observation that water in its solid state is less dense than water in its liquid state.
About the Ice-Water Anomaly
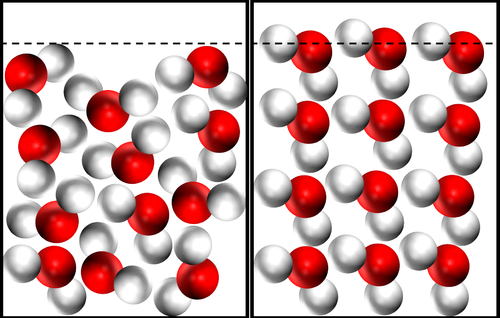
- For most substances the solid state is more dense than the liquid state. However, this is not the case for water.
- In liquid water the molecules are randomly arranged and close together. Whereas in solid water the molecules align with their Hydrogen atoms touching the Oxygen atoms of adjacent molecules.
| The same number of molecules takes up a larger volume in solid water, so Ice is less dense than liquid water. |
Key Stage 4
Meaning
The Ice-Water Anomaly is the observation that water in its solid state is less dense than water in its liquid state.
About the Ice-Water Anomaly
- For most substances the solid state is more dense than the liquid state. However, this is not the case for water.
- Water is known as a polar molecule which means one part is slightly positively charged and the other is slightly negatively charged. Since there is strong force of attraction pulling the electrons towards to Oxygen atom in water then the Oxygen becomes slightly negative and the Hydrogen atoms become slightly positively charged. This causes the molecules to line up with a Hydrogen of one molecule touching the Oxygen in another molecule.