Difference between revisions of "Particle Model"
(→Limitations of the Particle Model) |
(→Limitations of the Particle Model) |
||
| Line 55: | Line 55: | ||
|- | |- | ||
| style="height:20px; width:100px; text-align:left;" |There are no [[force]]s between [[particle]]s. | | style="height:20px; width:100px; text-align:left;" |There are no [[force]]s between [[particle]]s. | ||
| − | | style="height:20px; width:200px; text-align:left;" |[[Intermolecular Force]]s exist between [[molecule]]s. | + | | style="height:20px; width:200px; text-align:left;" |[[Intermolecular Force|Intermolecular force]]s exist between [[molecule]]s. |
| style="height:20px; width:300px; text-align:left;" | | | style="height:20px; width:300px; text-align:left;" | | ||
| − | : The strength of the [[Intermolecular Force|intermolecular forces]] affects whether a substance is [[solid]], [[liquid]] or [[gas]] at different [[temperature]]s. The stronger the [[Intermolecular Force|intermolecular forces]] the higher the [[temperature]] of its [[Melting Point|melting point]] and [[Boiling Point|boiling point]]. | + | : The strength of the [[Intermolecular Force|intermolecular forces]] affects whether a [[substance]] is [[solid]], [[liquid]] or [[gas]] at different [[temperature]]s. The stronger the [[Intermolecular Force|intermolecular forces]] the higher the [[temperature]] of its [[Melting Point|melting point]] and [[Boiling Point|boiling point]]. |
: The direction of the [[Intermolecular Force|intermolecular forces]] affects the way the [[particle]]s are arranged in a [[solid]]. | : The direction of the [[Intermolecular Force|intermolecular forces]] affects the way the [[particle]]s are arranged in a [[solid]]. | ||
|- | |- | ||
Revision as of 14:28, 19 December 2018
Contents
Key Stage 3
Meaning
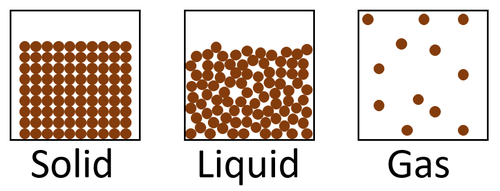
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
| A diagram showing the particle model for solids, liquids and gases. |
About The Particle Model
- The particle model explains the properties of solids, liquids and gases.
- The particle model can explain changes of state.
- Evidence of the particle model can be shown by pouring 50ml of pure water and 50ml of pure ethanol into a measuring cylinder. The solution is only 97ml because ethanol molecules are bigger than water molecules so the water molecules fit between the ethanol molecules like pouring 50ml of sand and 50ml of marbles into the same container. It will not make 100ml.
- Evidence of the particle model can be shown by observing Brownian Motion.
Key Stage 4
Meaning
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
About The Particle Model
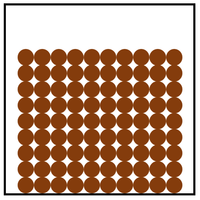
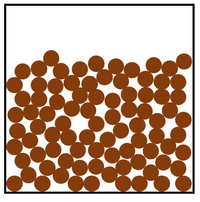
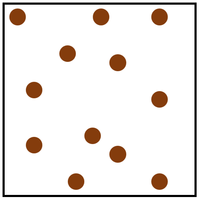
- The particle model describes how the particles that make a solid, liquid or gas are arranged and how they move.
| Diagram | Arrangement | Motion |
| In a solid the particles are in a regular arrangement and very close together. | In a solid the particles vibrate around fixed positions. | |
| In a liquid the particles are in a random arrangement with small gaps between them. | In a liquid the particles can slide past one another. | |
| In a gas the particles are in a random arrangement and spread far apart from one another. | In a gas the particles are free to move in all directions. |
Limitations of the Particle Model
- The particle model is not a complete explanation for the properties of a material. However, it is a useful approximation which can make predictions about the properties of solids, liquids and gases but is not perfect.
- The particle model only explains the properties of solids, liquids and gases but not why different materials are solid, liquid or gas at different temperatures.
The problems with the particle model are that it makes several assumptions which are not always the case:
| Assumption | Reality | Problem |
| Particles are spheres. | Particles are often molecules whose shape is not a sphere, some of which are long chains of atoms. |
|
| There are no forces between particles. | Intermolecular forces exist between molecules. |
|
| The size of all particles in a substance is the same. | A substance can be made of more than one different particle which have different sizes. |