Contents
Key Stage 3
Meaning
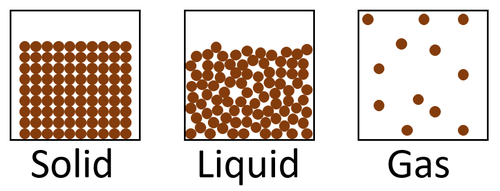
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
| A diagram showing the particle model for solids, liquids and gases. |
About The Particle Model
- The particle model explains the properties of solids, liquids and gases.
- The particle model can explain changes of state.
- Evidence of the particle model can be shown by pouring 50ml of pure water and 50ml of pure ethanol into a measuring cylinder. The solution is only 97ml because ethanol molecules are bigger than water molecules so the water molecules fit between the ethanol molecules like pouring 50ml of sand and 50ml of marbles into the same container. It will not make 100ml.
- Evidence of the particle model can be shown by observing Brownian Motion.
Key Stage 4
Meaning
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
About The Particle Model
- The particle model describes how the particles that make a solid, liquid or gas are arranged and how they move.
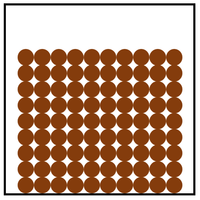
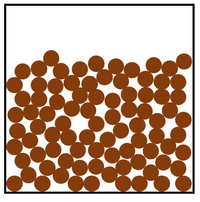
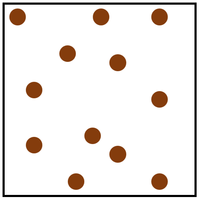
| Diagram | Arrangement | Motion |
| The particles are in a regular arrangement and very close together. | The particles vibrate around fixed positions. | |
| The particles are in a random arrangement with small gaps between them. | The particles can slide past one another. | |
| The particles are in a random arrangement and spread far apart from one another. | The particles are free to move in all directions. |