Difference between revisions of "Nuclear Fission"
| Line 8: | Line 8: | ||
: During '''nuclear fission''' [[neutron]]s are also [[emit]]ted. | : During '''nuclear fission''' [[neutron]]s are also [[emit]]ted. | ||
: '''Nuclear fission''' [[Energy Transfer|transfers]] [[energy]] from the [[Nuclear Potential Energy Store|nuclear potential energy store]] into the [[Thermal Energy Store|thermal energy store]] of the [[material]] and the surroundings. | : '''Nuclear fission''' [[Energy Transfer|transfers]] [[energy]] from the [[Nuclear Potential Energy Store|nuclear potential energy store]] into the [[Thermal Energy Store|thermal energy store]] of the [[material]] and the surroundings. | ||
| + | : In a '''nuclear fission reaction''' the products have less [[mass]] than the reactants as some of the [[Rest Mass Energy|rest mass]] is converted into [[energy]] in the process. | ||
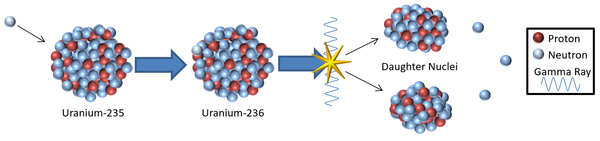
: '''Nuclear fission''' can be induced in a [[material]] by bombarding [[massive]] [[Atomic Nucleus|nuclei]] with [[neutron]]s. If a [[neutron]] is captured by the [[Atomic Nucleus|nucleus]] it becomes so unstable that it splits in two. | : '''Nuclear fission''' can be induced in a [[material]] by bombarding [[massive]] [[Atomic Nucleus|nuclei]] with [[neutron]]s. If a [[neutron]] is captured by the [[Atomic Nucleus|nucleus]] it becomes so unstable that it splits in two. | ||
: The [[neutron]]s used to induce '''fission''' must have a low [[energy]] to be captured by a [[Atomic Nucleus|nucleus]] otherwise the [[neutron]]s will just pass straight through without being captured. [[Neutron]]s with the right amount of [[energy]] to be captured are called [[Thermal Neutron|thermal neutron]]s because they have a similar [[energy]] to [[molecule]]s in the [[air]] at [[Room Temperature|room temperature]]. | : The [[neutron]]s used to induce '''fission''' must have a low [[energy]] to be captured by a [[Atomic Nucleus|nucleus]] otherwise the [[neutron]]s will just pass straight through without being captured. [[Neutron]]s with the right amount of [[energy]] to be captured are called [[Thermal Neutron|thermal neutron]]s because they have a similar [[energy]] to [[molecule]]s in the [[air]] at [[Room Temperature|room temperature]]. | ||
Revision as of 13:38, 11 March 2019
Key Stage 4
Meaning
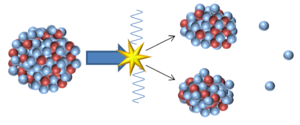
An model of nuclear fission.
Nuclear fission is a process in which a large unstable nucleus splits into two more stable nuclei.
About Nuclear Fission
- Nuclear fission occurs when a massive nucleus is so unstable that it splits in two.
- During nuclear fission neutrons are also emitted.
- Nuclear fission transfers energy from the nuclear potential energy store into the thermal energy store of the material and the surroundings.
- In a nuclear fission reaction the products have less mass than the reactants as some of the rest mass is converted into energy in the process.
- Nuclear fission can be induced in a material by bombarding massive nuclei with neutrons. If a neutron is captured by the nucleus it becomes so unstable that it splits in two.
- The neutrons used to induce fission must have a low energy to be captured by a nucleus otherwise the neutrons will just pass straight through without being captured. Neutrons with the right amount of energy to be captured are called thermal neutrons because they have a similar energy to molecules in the air at room temperature.
| A model showing a possible mechanism for induced nuclear fission resulting from the capture of a thermal neutron.
\({}_{92}^{235}U + {}_{0}^{1}n \rightarrow {}_{92}^{236}U \rightarrow {}_{36}^{85}Kr + {}_{56}^{148}Ba + 3{}_{0}^{1}n\) |
- If there is enough of an unstable isotope in a material a single nuclear fission can trigger a nuclear chain reaction in which the neutrons produced from the initial fission event can cause the fission of more than one other unstable isotope.
- A nuclear fission chain reaction is used in both nuclear bombs and nuclear reactors in nuclear power stations.