Difference between revisions of "Group 2"
(→Key Stage 4) |
|||
| Line 39: | Line 39: | ||
:*[[Barium]] | :*[[Barium]] | ||
:*[[Radium]] | :*[[Radium]] | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Alkali earth metals, page 133, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Alkali earth metals; flame tests, page 274-5, GCSE Chemistry; Student Book, Collins, AQA'] | ||
Revision as of 18:06, 27 October 2019
Contents
Key Stage 3
Meaning
Group 2 are elements on the Periodic Table with only 2 electrons in their outer shell.
About Group 2
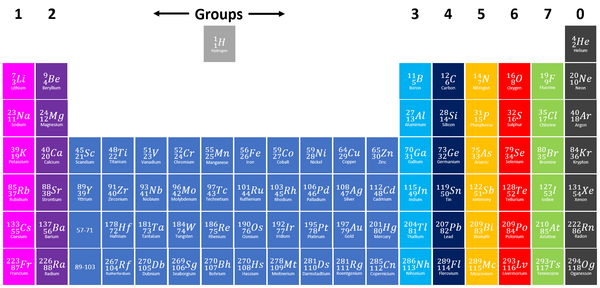
| Group 2 elements are shown in dark purple at the left of the Periodic Table. |
Key Stage 4
Meaning
Group 2 are elements on the Periodic Table with only 2 electrons in their outer shell.
About Group 2
- Group 2 atoms can lose 2 electrons to form positive ions with a charge of +2.
- Group 2 elements are known as the Alkali Earth Metals.
| Group 2 elements are shown in dark purple at the left of the Periodic Table. |