Difference between revisions of "Emission Spectra"
| Line 17: | Line 17: | ||
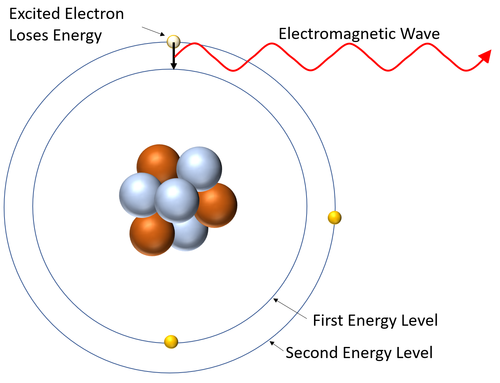
| style="height:20px; width:200px; text-align:center;" |This [[diagram]] shows an [[excited]] [[electron]] losing [[energy]] by [[emit]]ting an [[Electromagnetic Wave|electromagnetic wave]]. As it does this the [[electron]] falls back down to a lower [[Energy Level|energy level]]. | | style="height:20px; width:200px; text-align:center;" |This [[diagram]] shows an [[excited]] [[electron]] losing [[energy]] by [[emit]]ting an [[Electromagnetic Wave|electromagnetic wave]]. As it does this the [[electron]] falls back down to a lower [[Energy Level|energy level]]. | ||
|} | |} | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Emission spectra, pages 214-15, GCSE Chemistry, Hodder, AQA ''] | ||
Revision as of 11:11, 4 November 2019
Key Stage 4
Meaning
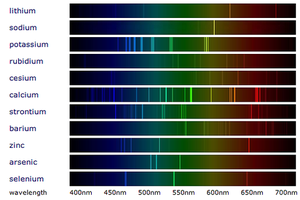
Emission spectra are the specific wavelengths of light emitted by the electrons in atoms as they lose energy.
About Emission Spectra
- An emission spectrum is made by providing energy to a material and focusing any light emitted through a prism to separate the colours.
- The spectrum of white light is a continuous change of colours with all wavelengths having the same intensity.
- An emission spectrum is a set of specific wavelengths with a high intensity. This appears as bright lines of colour on a spectrum.
- A emission spectrum is created when excited electrons (electrons in high energy levels) lose energy and fall to a lower energy level emitting a specific wavelength of electromagnetic wave when they do.
- The wavelengths of electromagnetic wave depend on the energy difference between the energy levels in atoms.
| This diagram shows an excited electron losing energy by emitting an electromagnetic wave. As it does this the electron falls back down to a lower energy level. |