Difference between revisions of "Polymer"
| Line 101: | Line 101: | ||
===Extra Information=== | ===Extra Information=== | ||
{{#ev:youtube|https://www.youtube.com/watch?v=UwRVj9rz2QQ}} | {{#ev:youtube|https://www.youtube.com/watch?v=UwRVj9rz2QQ}} | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158754/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158754&linkCode=as2&tag=nrjc-21&linkId=27ad53b0283feeff7fc5ae04a9e205f443 ''Polymer, page 244, GCSE Biology; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer, pages 62, 74-5, 226-7, 246-9, 342-3, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; addition, pages 75, 226-7, 246-7, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; condensation, pages 75, 226-7, 247-9, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; cross-linking, page 343, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; natural, pages 226-7, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; poly(ethene), pages 74-5, 226, 246-7, 254-5, 257, 343, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; poly(propene), pages 75, 246-7, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; polypeptide, page 251, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; polysaccharide, pages 252-3, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; PVC, pages 74-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; thermosetting, page 343, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Polymer; thermosoftening, page 343, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Revision as of 21:57, 10 November 2019
Contents
Key Stage 3
Meaning
A polymer is a long molecule made by reacting together several smaller molecules.
About Polymers
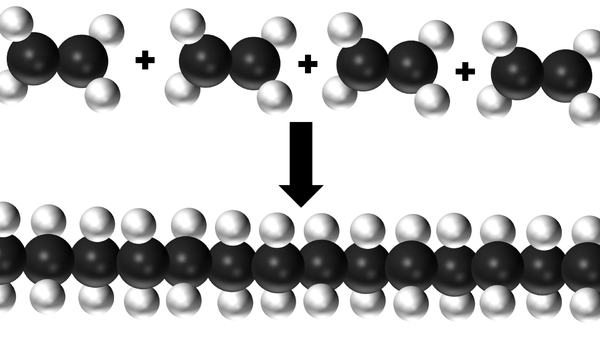
| This diagram shows several Ethene molecules reacting together to make a polythene molecule. |
Different polymers can have different properties but generally polymers are:
- Easily molded into shape.
- Solid at room temperature.
- Plastic in behaviour.
- An Electrical Insulator.
| Polymer | Properties | Application |
| Polythene | Plastic, Strong, Ductile | Shopping bags |
| PVC | Plastic, Electrical Insulator, Strong | Covering of electrical wires. |
| Nylon | Plastic, Strong, Flexible, Ductile. | Clothing |
Key Stage 4
Meaning
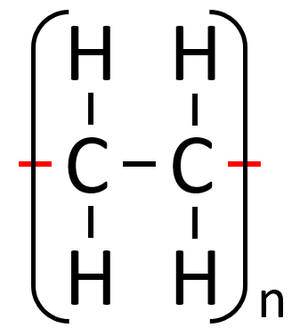
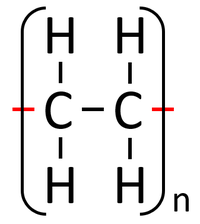
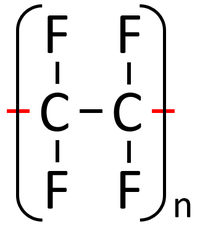
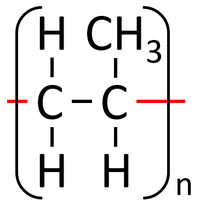
A polymer is represented by showing the part that is repeated over and over. The 'n' represents the fact that it is repeated many times.
A polymer is a large molecule made of many identical smaller molecules called monomers.
About Polymers
- Polymers are held together by covalent bonds.
- The monomers which make up a polymer are simple covalent molecules.
- Most polymers are made of chains of Carbon atoms since they can from up to 4 covalent bonds with adjacent atoms. However, some can be made from Silicon, which can also form 4 covalent bonds.
Examples
| Polythene is a polymer made by reacting thousands of Ethene monomers. | PolyTetraFluoroEthene is a polymer made by reacting thousands of TetraFluoroEthene monomers. | PolyPropene is a polymer made by reacting thousands of Propene monomers. |
Applications and Properties
| Application | Polymer | Properties |
| Windows | Polymethylmethacrylate | Transparent - Light can pass through.
Flexible - Does not break or shatter when a force is applied. |
| Covering electrical wires | PVC (Polychloroethene) | Plastic - Can be molded into shape.
Electrical Insulator - Prevents electricity leaving the wires. Strong - Is not broken easily when a force is applied. |
| Shopping bags | Polythene | Plastic - Can be molded into shape.
Strong - A very thin bag can hold a large weight. Ductile - Can be stretched into shape. Flexible - Can be bent without breaking. |
| Clothes | Nylon | Plastic - Can be molded into shape.
Strong - A very thin fiber can hold a large weight. Ductile - Can be stretched into fibers. Flexible - Can be bent without breaking. |
Extra Information
References
AQA
- Polymer, page 244, GCSE Biology; Student Book, Collins, AQA
- Polymer, pages 62, 74-5, 226-7, 246-9, 342-3, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; addition, pages 75, 226-7, 246-7, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; condensation, pages 75, 226-7, 247-9, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; cross-linking, page 343, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; natural, pages 226-7, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; poly(ethene), pages 74-5, 226, 246-7, 254-5, 257, 343, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; poly(propene), pages 75, 246-7, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; polypeptide, page 251, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; polysaccharide, pages 252-3, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; PVC, pages 74-5, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; thermosetting, page 343, GCSE Chemistry; Student Book, Collins, AQA
- Polymer; thermosoftening, page 343, GCSE Chemistry; Student Book, Collins, AQA