Difference between pages "Ore" and "Nuclear Fission"
(Difference between pages)| Line 1: | Line 1: | ||
| − | ==Key Stage | + | ==Key Stage 4== |
===Meaning=== | ===Meaning=== | ||
| − | [[File: | + | [[File:Fission2.png|right|300px|thumb|An [[model]] of '''nuclear fission'''.]] |
| − | + | '''Nuclear fission''' is a process in which a large [[Unstable Isotope|unstable]] [[Atomic Nucleus|nucleus]] splits into two more [[Stable Isotope|stable]] [[Atomic Nucleus|nuclei]]. | |
| − | ===About | + | ===About Nuclear Fission=== |
| − | : [[ | + | : '''Nuclear fission''' occurs when a [[massive]] [[Atomic Nucleus|nucleus]] is so [[Unstable Isotope|unstable]] that it splits in two. |
| − | : | + | : During '''nuclear fission''' [[neutron]]s are also [[emit]]ted. |
| + | : '''Nuclear fission''' [[Energy Transfer|transfers]] [[energy]] from the [[Nuclear Potential Energy Store|nuclear potential energy store]] into the [[Thermal Energy Store|thermal energy store]] of the [[material]] and the surroundings. | ||
| + | : In a '''nuclear fission reaction''' the products have less [[mass]] than the reactants as some of the [[mass]] is converted into [[energy]] in the process due to the [[Mass-Energy Equivalence]]. | ||
| + | : '''Nuclear fission''' can be induced in a [[material]] by bombarding [[massive]] [[Atomic Nucleus|nuclei]] with [[neutron]]s. If a [[neutron]] is captured by the [[Atomic Nucleus|nucleus]] it becomes so unstable that it splits in two. | ||
| + | : The [[neutron]]s used to induce '''fission''' must have a low [[energy]] to be captured by a [[Atomic Nucleus|nucleus]] otherwise the [[neutron]]s will just pass straight through without being captured. [[Neutron]]s with the right amount of [[energy]] to be captured are called [[Thermal Neutron|thermal neutron]]s because they have a similar [[energy]] to [[molecule]]s in the [[air]] at [[Room Temperature|room temperature]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
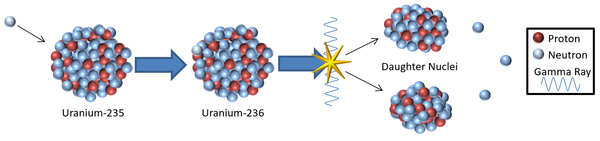
| + | |[[File:InducedFission.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:600px; text-align:left;" |A [[model]] showing a possible mechanism for induced '''nuclear fission''' resulting from the capture of a [[Thermal Neutron|thermal neutron]]. | ||
| − | + | <math>{}_{92}^{235}U + {}_{0}^{1}n \rightarrow {}_{92}^{236}U \rightarrow {}_{36}^{85}Kr + {}_{56}^{148}Ba + 3{}_{0}^{1}n</math> | |
| − | + | |} | |
| − | |||
| − | + | : If there is enough of an [[Unstable Isotope|unstable isotope]] in a [[material]] a single '''nuclear fission''' can trigger a [[Nuclear Chain Reaction|nuclear chain reaction]] in which the [[neutron]]s produced from the initial '''fission''' event can cause the '''fission''' of more than one other [[Unstable Isotope|unstable isotope]]. | |
| − | : | + | : A [[Nuclear Fission|nuclear fission]] [[Nuclear Chain Reaction|chain reaction]] is used in both [[Nuclear Bomb|nuclear bombs]] and [[Nuclear Fission Reactor|nuclear reactors]] in [[Nuclear Power|nuclear power stations]]. |
| − | : | ||
| − | |||
===References=== | ===References=== | ||
====AQA==== | ====AQA==== | ||
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Nuclear fission, page 140, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] |
| − | + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac337 ''Nuclear fission, page 49, GCSE Physics; The Revision Guide, CGP, AQA ''] | |
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Nuclear fission, pages 104-105, GCSE Physics; Third Edition, Oxford University Press, AQA\ ''] |
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Nuclear fission, pages 106-7, GCSE Physics, Hodder, AQA ''] |
| − | |||
| − | |||
| − | |||
| − | :[https://www.amazon.co.uk/gp/product/ | ||
====Edexcel==== | ====Edexcel==== | ||
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Nuclear fission, page 166, GCSE Combined Science, Pearson Edexcel ''] |
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Nuclear fission, page 22, GCSE Chemistry, Pearson, Edexcel ''] |
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Nuclear fission, page 57, GCSE Physics; The Revision Guide, CGP, Edexcel ''] |
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Nuclear fission, pages 110, 112-113, GCSE Physics, Pearson Edexcel ''] |
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Nuclear fission; power generation, page 113, GCSE Physics, Pearson Edexcel ''] | ||
====OCR==== | ====OCR==== | ||
| − | :[https://www.amazon.co.uk/gp/product/ | + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Nuclear fission, pages 184-185, Gateway GCSE Physics, Oxford, OCR ''] |
| − | + | ||
| − | + | ==Key Stage 5== | |
| + | ===Meaning=== | ||
| + | [[Fission]] is the splitting of a heavy [[Atomic Nucleus|nucleus]] into two approximately equal fragments, releasing [[energy]]. | ||
| + | |||
| + | ===About Fission=== | ||
| + | |||
| + | [[Nuclear fission]] can be spontaneous or [[Electromagnetic Induction|induced]] by the absorption of a [[neutron]]. | ||
| + | When [[uranium-235]] ]] or [[plutonium-239]] absorbs a [[neutron]], it becomes unstable and splits into two smaller [[Atomic Nucleus|nuclei]] along with additional [[neutron]]s and a large amount of [[energy]]. | ||
| + | The released [[neutron]]s can induce further [[Nuclear Fission|fission]] reactions, leading to a chain reaction. | ||
| + | Fission releases a significant amount of [[energy]], primarily in the form of kinetic [[energy]] of the [[Nuclear Fission|fission]] fragments. | ||
| + | Fission is used in [[nuclear reactors]] to generate [[electricity]] and in [[nuclear weapons]]. | ||
Revision as of 10:28, 30 May 2024
Contents
Key Stage 4
Meaning
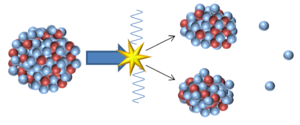
Nuclear fission is a process in which a large unstable nucleus splits into two more stable nuclei.
About Nuclear Fission
- Nuclear fission occurs when a massive nucleus is so unstable that it splits in two.
- During nuclear fission neutrons are also emitted.
- Nuclear fission transfers energy from the nuclear potential energy store into the thermal energy store of the material and the surroundings.
- In a nuclear fission reaction the products have less mass than the reactants as some of the mass is converted into energy in the process due to the Mass-Energy Equivalence.
- Nuclear fission can be induced in a material by bombarding massive nuclei with neutrons. If a neutron is captured by the nucleus it becomes so unstable that it splits in two.
- The neutrons used to induce fission must have a low energy to be captured by a nucleus otherwise the neutrons will just pass straight through without being captured. Neutrons with the right amount of energy to be captured are called thermal neutrons because they have a similar energy to molecules in the air at room temperature.
| A model showing a possible mechanism for induced nuclear fission resulting from the capture of a thermal neutron.
\({}_{92}^{235}U + {}_{0}^{1}n \rightarrow {}_{92}^{236}U \rightarrow {}_{36}^{85}Kr + {}_{56}^{148}Ba + 3{}_{0}^{1}n\) |
- If there is enough of an unstable isotope in a material a single nuclear fission can trigger a nuclear chain reaction in which the neutrons produced from the initial fission event can cause the fission of more than one other unstable isotope.
- A nuclear fission chain reaction is used in both nuclear bombs and nuclear reactors in nuclear power stations.
References
AQA
- Nuclear fission, page 140, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Nuclear fission, page 49, GCSE Physics; The Revision Guide, CGP, AQA
- Nuclear fission, pages 104-105, GCSE Physics; Third Edition, Oxford University Press, AQA\
- Nuclear fission, pages 106-7, GCSE Physics, Hodder, AQA
Edexcel
- Nuclear fission, page 166, GCSE Combined Science, Pearson Edexcel
- Nuclear fission, page 22, GCSE Chemistry, Pearson, Edexcel
- Nuclear fission, page 57, GCSE Physics; The Revision Guide, CGP, Edexcel
- Nuclear fission, pages 110, 112-113, GCSE Physics, Pearson Edexcel
- Nuclear fission; power generation, page 113, GCSE Physics, Pearson Edexcel
OCR
Key Stage 5
Meaning
Fission is the splitting of a heavy nucleus into two approximately equal fragments, releasing energy.
About Fission
Nuclear fission can be spontaneous or induced by the absorption of a neutron. When uranium-235 ]] or plutonium-239 absorbs a neutron, it becomes unstable and splits into two smaller nuclei along with additional neutrons and a large amount of energy. The released neutrons can induce further fission reactions, leading to a chain reaction. Fission releases a significant amount of energy, primarily in the form of kinetic energy of the fission fragments. Fission is used in nuclear reactors to generate electricity and in nuclear weapons.