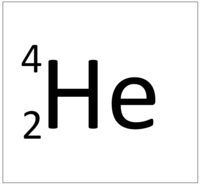Difference between revisions of "Calculating the Numbers of Protons, Neutrons and Electrons"
| Line 6: | Line 6: | ||
===About Calculating the Numbers of Protons, Neutrons and Electrons=== | ===About Calculating the Numbers of Protons, Neutrons and Electrons=== | ||
: The number of [[proton]]s in an [[atom]] or [[ion]] is given by the [[Atomic Number]]. | : The number of [[proton]]s in an [[atom]] or [[ion]] is given by the [[Atomic Number]]. | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Helium''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Lithium''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Beryllium''' | ||
| + | |- | ||
| + | |[[File:Hydrogen.png|center|150px]] | ||
| + | |[[File:Helium.png|center|150px]] | ||
| + | |[[File:Lithium.png|center|200px]] | ||
| + | |[[File:Beryllium.png|center|200px]] | ||
| + | |- | ||
| + | |[[File:HydrogenSymbol.png|center|200px]] | ||
| + | |[[File:HeliumSymbol.png|center|200px]] | ||
| + | |[[File:LithiumSymbol.png|center|200px]] | ||
| + | |[[File:BerylliumSymbol.png|center|200px]] | ||
| + | |- | ||
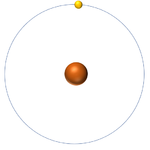
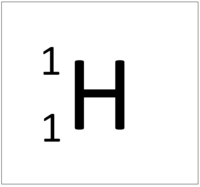
| + | | style="height:20px; width:200px; text-align:center;" |[[Hydrogen]] has an [[Atomic Number]] of 1 so it has 1 [[proton]]. | ||
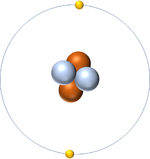
| + | | style="height:20px; width:200px; text-align:center;" |[[Helium]] has an [[Atomic Number]] of 2 so it has 2 [[proton]]s. | ||
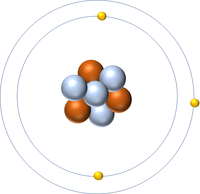
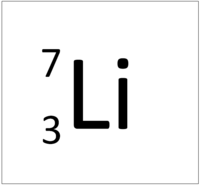
| + | | style="height:20px; width:200px; text-align:center;" |[[Lithium]] has an [[Atomic Number]] of 3 so it has 3 [[proton]]s. | ||
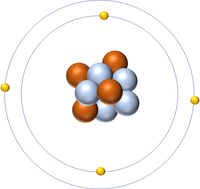
| + | | style="height:20px; width:200px; text-align:center;" |[[Beryllium]] has an [[Atomic Number]] of 4 so it has 4 [[proton]]s. | ||
| + | |} | ||
| + | |||
: The number of [[neutron]]s can be found by subtracting the [[Atomic Number]] from the [[Relative Atomic Mass]]. | : The number of [[neutron]]s can be found by subtracting the [[Atomic Number]] from the [[Relative Atomic Mass]]. | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Hydrogen''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Helium''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Lithium''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Beryllium''' | ||
| + | |- | ||
| + | |[[File:HydrogenSymbol.png|center|200px]] | ||
| + | |[[File:HeliumSymbol.png|center|200px]] | ||
| + | |[[File:LithiumSymbol.png|center|200px]] | ||
| + | |[[File:BerylliumSymbol.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:left;" |This [[atom]] has an [[Atomic Number]] (Z) of 1 and a [[Relative Atomic Mass]] (A) of 1. | ||
| + | |||
| + | Number of [[neutron]]s = A - Z | ||
| + | |||
| + | Number of [[neutron]]s = 1 - 1 | ||
| + | |||
| + | Number of [[neutron]]s = 0 | ||
| + | | style="height:20px; width:200px; text-align:left;" |This [[atom]] has an [[Atomic Number]] (Z) of 2 and a [[Relative Atomic Mass]] (A) of 4. | ||
| + | |||
| + | Number of [[neutron]]s = A - Z | ||
| + | |||
| + | Number of [[neutron]]s = 4 - 2 | ||
| + | |||
| + | Number of [[neutron]]s = 2 | ||
| + | | style="height:20px; width:200px; text-align:left;" |This [[atom]] has an [[Atomic Number]] (Z) of 3 and a [[Relative Atomic Mass]] (A) of 7. | ||
| + | |||
| + | Number of [[neutron]]s = A - Z | ||
| + | |||
| + | Number of [[neutron]]s = 7 - 3 | ||
| + | |||
| + | Number of [[neutron]]s = 4 | ||
| + | | style="height:20px; width:200px; text-align:left;" |This [[atom]] has an [[Atomic Number]] (Z) of 4 and a [[Relative Atomic Mass]] (A) of 9. | ||
| + | |||
| + | Number of [[neutron]]s = A - Z | ||
| + | |||
| + | Number of [[neutron]]s = 9 - 4 | ||
| + | |||
| + | Number of [[neutron]]s = 5 | ||
| + | |} | ||
: The number of [[electron]] in an [[ion]] can be found using the [[Atomic Number]] (which is the same as the [[Relative Atomic Charge]] of the [[Atomic Nucleus|nucleus]]) and subtracting the [[charge]] of the [[ion]]. | : The number of [[electron]] in an [[ion]] can be found using the [[Atomic Number]] (which is the same as the [[Relative Atomic Charge]] of the [[Atomic Nucleus|nucleus]]) and subtracting the [[charge]] of the [[ion]]. | ||
Revision as of 14:30, 26 November 2018
Key Stage 4
Meaning
File:Picture.png
Text
Calculting the numbers of protons, neutrons, and electrons can be done from information about the Atomic Number, Relative Atomic Mass and Relative Atomic Charge of a particle.
About Calculating the Numbers of Protons, Neutrons and Electrons
- The number of protons in an atom or ion is given by the Atomic Number.
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has an Atomic Number of 1 so it has 1 proton. | Helium has an Atomic Number of 2 so it has 2 protons. | Lithium has an Atomic Number of 3 so it has 3 protons. | Beryllium has an Atomic Number of 4 so it has 4 protons. |
- The number of neutrons can be found by subtracting the Atomic Number from the Relative Atomic Mass.
| Hydrogen | Helium | Lithium | Beryllium |
| This atom has an Atomic Number (Z) of 1 and a Relative Atomic Mass (A) of 1.
Number of neutrons = A - Z Number of neutrons = 1 - 1 Number of neutrons = 0 |
This atom has an Atomic Number (Z) of 2 and a Relative Atomic Mass (A) of 4.
Number of neutrons = A - Z Number of neutrons = 4 - 2 Number of neutrons = 2 |
This atom has an Atomic Number (Z) of 3 and a Relative Atomic Mass (A) of 7.
Number of neutrons = A - Z Number of neutrons = 7 - 3 Number of neutrons = 4 |
This atom has an Atomic Number (Z) of 4 and a Relative Atomic Mass (A) of 9.
Number of neutrons = A - Z Number of neutrons = 9 - 4 Number of neutrons = 5 |
- The number of electron in an ion can be found using the Atomic Number (which is the same as the Relative Atomic Charge of the nucleus) and subtracting the charge of the ion.