Difference between revisions of "Outer Shell"
(Created page with "==Key Stage 3== ===Meaning=== right|300px|thumb|A [[diagram showing the three '''electron shells''' of a Sodium atom. The '''outer shell''' i...") |
|||
| Line 6: | Line 6: | ||
===About the Outer Shell=== | ===About the Outer Shell=== | ||
: The '''outer shell''' is the only [[Electron Orbital|electron orbital]] that is affected in [[Chemical Reaction|chemical reactions]]. | : The '''outer shell''' is the only [[Electron Orbital|electron orbital]] that is affected in [[Chemical Reaction|chemical reactions]]. | ||
| − | : The number of [[electron]]s in the last ''' | + | : The number of [[electron]]s in the last '''Outer Shell''' determines the [[Group (Chemistry)|Group]] on the [[Periodic Table]]. |
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
Revision as of 12:39, 6 December 2018
Key Stage 3
Meaning
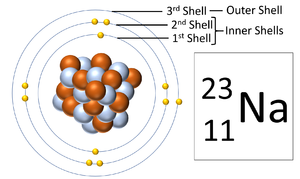
A diagram showing the three electron shells of a Sodium atom. The outer shell is the electron orbital furthest from the nucleus.
The outer shell is the electron orbital furthest from the nucleus of an atom.
About the Outer Shell
- The outer shell is the only electron orbital that is affected in chemical reactions.
- The number of electrons in the last Outer Shell determines the Group on the Periodic Table.
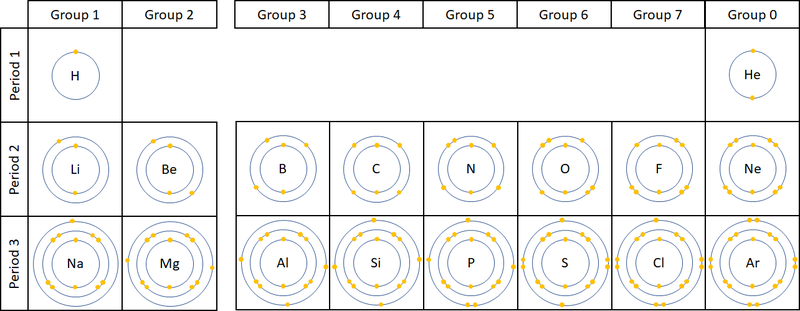
| This diagram shows that the number of electrons in the outer shell determines which group the elements have been placed into in the Periodic Table. NB Group 0 used to be called Group 8 but this caused confusion because most elements in Group 8 have 8 electrons in their Outer Shell but Helium only has 2, so it was renamed Group 0. |