Difference between revisions of "Giant Ionic Structure"
(Created page with "==Key Stage 4== ===Meaning=== '''Giant ionic structures''' are large molecules in which the atoms are held together by ionic bonds. ===About Giant Ioni...") |
|||
| Line 16: | Line 16: | ||
===Bulk Properties=== | ===Bulk Properties=== | ||
| + | : '''Giant ionic structures''' are poor [[Electrical Conductor|electrical conductors]] because the [[ion]]s are not free to move. | ||
| + | : Most '''giant ionic structures''' can be broken down and [[dissolve]]d in [[water]]. | ||
| + | : [[Giant Ionic Structure|Giant ionic structures]] have high [[Melting Point|melting points]] due to the strong [[Electrostatic Force|electrostatic force]] between the [[ion]]s. | ||
Revision as of 14:24, 28 December 2018
Contents
Key Stage 4
Meaning
Giant ionic structures are large molecules in which the atoms are held together by ionic bonds.
About Giant Ionic Structures
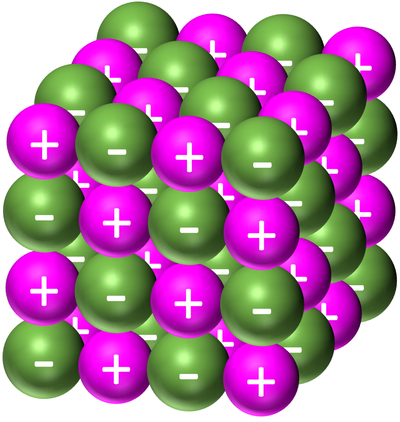
- Giant ionic structures are molecules made of a large number of metal and non-metal ions joined by ionic bonds.
- The ions in a giant ionic structure are arranged in a regular lattice (a repeating pattern of elements.
Examples
| Sodium Chloride forms a giant ionic structure. The Sodium is represented by the light purple positive ions and the Chloride is represented by the green negative ions. |
Bulk Properties
- Giant ionic structures are poor electrical conductors because the ions are not free to move.
- Most giant ionic structures can be broken down and dissolved in water.
- Giant ionic structures have high melting points due to the strong electrostatic force between the ions.