Difference between revisions of "Dot and Cross Diagram"
(Created page with "==Key Stage 4== ===Meaning=== A '''dot and cross diagram''' is a diagram used to show how electrons from the outer shells of atoms are shared or tr...") |
|||
| Line 2: | Line 2: | ||
===Meaning=== | ===Meaning=== | ||
A '''dot and cross diagram''' is a [[diagram]] used to show how [[electron]]s from the [[Outer Shell|outer shells]] of [[atom]]s are shared or transferred in a [[Chemical Bond|chemical bond]]. | A '''dot and cross diagram''' is a [[diagram]] used to show how [[electron]]s from the [[Outer Shell|outer shells]] of [[atom]]s are shared or transferred in a [[Chemical Bond|chemical bond]]. | ||
| + | |||
| + | ===About Dot and Cross Diagrams=== | ||
| + | : '''Dot and cross diagrams''' can be used to represent [[Covalent Bond|covalent bonds]] and [[Ionic Bond|ionic bonds]]. | ||
| + | |||
| + | ===Examples=== | ||
| + | {| class="wikitable" | ||
| + | |+ Covalent Bonds | ||
| + | |- | ||
| + | |[[File:OxygenDotandCrossDiagram.png|center|175px]] | ||
| + | |[[File:NitrogenDotandCrossDiagram.png|center|175px]] | ||
| + | |[[File:CarbonDioxideDotandCrossDiagram.png|center|250px]] | ||
| + | |- | ||
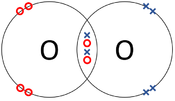
| + | | style="height:20px; width:175px; text-align:center;" |The two [[Oxygen]] [[atom]]s each share two of their [[electron]]s with one another. | ||
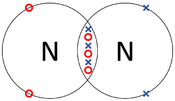
| + | | style="height:20px; width:175px; text-align:center;" |The two [[Nitrogen]] [[atom]]s each share three of their [[electron]]s with one another. | ||
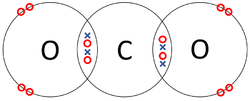
| + | | style="height:20px; width:250px; text-align:center;" |Each [[Oxygen]] shares two of its [[electron]]s with the [[Carbon]] [[atom]] while the [[Carbon]] [[atom]] shares two [[electron]]s with each [[Oxygen]] [[atom]]. | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ Ionic Bonds | ||
| + | |- | ||
| + | |[[File:LithiumFluorideDotandCrossDiagram.png|center|200px]] | ||
| + | |[[File:MagnesiumOxideDotandCrossDiagram.png|center|200px]] | ||
| + | |[[File:BerylliumFluorideDotandCrossDiagram.png|center|200px]] | ||
| + | |- | ||
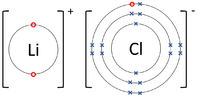
| + | | style="height:20px; width:200px; text-align:center;" |The [[Lithium]] [[atom]] donates an [[electron]] from its [[Outer Shell|outer shell]] to the [[Outer Shell|outer shell]] of the [[Fluorine]] [[atom]]. | ||
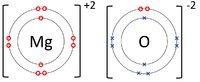
| + | | style="height:20px; width:200px; text-align:center;" |The [[Magnesium]] [[atom]] donates two [[electron]]s from its [[Outer Shell|outer shell]] to the [[Outer Shell|outer shell]] of the [[Oxygen]] [[atom]]. | ||
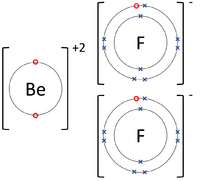
| + | | style="height:20px; width:200px; text-align:center;" |The [[Beryllium]] [[atom]] donates two [[electron]]s from its [[Outer Shell|outer shell]] to the [[Outer Shell|outer shells]] of each [[Fluorine]] [[atom]]. | ||
| + | |} | ||
Revision as of 17:18, 31 December 2018
Key Stage 4
Meaning
A dot and cross diagram is a diagram used to show how electrons from the outer shells of atoms are shared or transferred in a chemical bond.
About Dot and Cross Diagrams
- Dot and cross diagrams can be used to represent covalent bonds and ionic bonds.
Examples
| The two Oxygen atoms each share two of their electrons with one another. | The two Nitrogen atoms each share three of their electrons with one another. | Each Oxygen shares two of its electrons with the Carbon atom while the Carbon atom shares two electrons with each Oxygen atom. |
| The Lithium atom donates an electron from its outer shell to the outer shell of the Fluorine atom. | The Magnesium atom donates two electrons from its outer shell to the outer shell of the Oxygen atom. | The Beryllium atom donates two electrons from its outer shell to the outer shells of each Fluorine atom. |