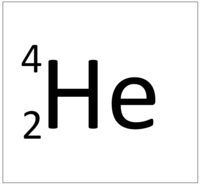Difference between revisions of "Relative Atomic Mass"
(→Meaning) |
(→About Relative Atomic Mass) |
||
| Line 41: | Line 41: | ||
===About Relative Atomic Mass=== | ===About Relative Atomic Mass=== | ||
| − | : The '''relative atomic mass''' in [[gram]]s on the [[Periodic Table]] tells the [[mass]] of a [[mole]] of the [[element]]. | + | : The '''relative atomic mass''' in [[gram]]s on the [[Periodic Table]] tells the [[mass]] of a [[mole]] of the [[element]]. A [[mole]] of the [[element]] is [[Avagadro Constant|6.02x10<sup>23</sup>]] [[atom]]s of that [[element]]. |
| − | |||
: Two [[atom]]s of the same [[element]] may have the same [[Atomic Number]] but a different [[Relative Atomic Mass]] depending on the number of [[neutron]]s in the [[nucleus]]. [[Element]]s with different '''mass numbers''' are called [[isotope]]s. | : Two [[atom]]s of the same [[element]] may have the same [[Atomic Number]] but a different [[Relative Atomic Mass]] depending on the number of [[neutron]]s in the [[nucleus]]. [[Element]]s with different '''mass numbers''' are called [[isotope]]s. | ||
: The '''relative atomic mass''' is not affected by the number of [[electron]]s. | : The '''relative atomic mass''' is not affected by the number of [[electron]]s. | ||
Revision as of 18:55, 2 January 2019
Contents
Key Stage 3
Meaning
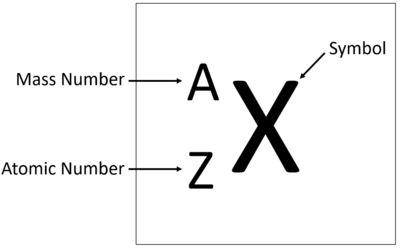
An element tile showing the mass number.
The Atomic Mass or mass number is the number of nucleons (protons + neutrons) in an atom.
About The Atomic Mass
- Two atoms of the same element may have the same Atomic Number but a different Atomic Mass depending on the number of neutrons in the nucleus. Elements with different mass numbers are called isotopes.
- The atomic mass is not affected by the number of electrons.
- Only the particles in the nucleus affect the atomic mass.
Examples
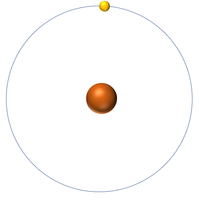
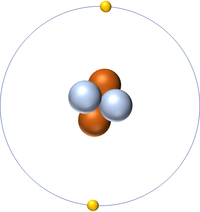
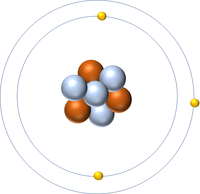
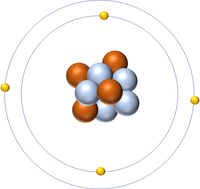
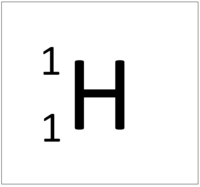
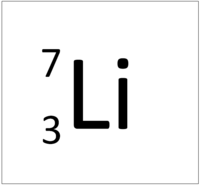
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has one nucleon so it has an atomic mass of 1. | Helium has four nucleons so it has an atomic mass of 4. | Lithium has seven nucleons so it has an atomic mass of 7. | Beryllium has nine nucleons so it has an atomic mass of 9. |
Key Stage 3
Meaning

An element tile showing the mass number.
The relative atomic mass or mass number of an element is the number of nucleons (protons + neutrons) in an atom.
The relative atomic mass in grams is also the mass of one mole or 6.02x1023 atoms of the element.
About Relative Atomic Mass
- The relative atomic mass in grams on the Periodic Table tells the mass of a mole of the element. A mole of the element is 6.02x1023 atoms of that element.
- Two atoms of the same element may have the same Atomic Number but a different Relative Atomic Mass depending on the number of neutrons in the nucleus. Elements with different mass numbers are called isotopes.
- The relative atomic mass is not affected by the number of electrons.
- Only the particles in the nucleus affect the relative atomic mass.
Examples
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen has one nucleon so it has an atomic mass of 1 and 6.02x1023 (or 1 mole of) atoms of Hydrogen have a mass of 1g. | Helium has four nucleons so it has an atomic mass of 4 and 6.02x1023 (or 1 mole of) atoms of Helium have a mass of 4g. | Lithium has seven nucleons so it has an atomic mass of 7 and 6.02x1023 (or 1 mole of) atoms of Lithium have a mass of 7g. | Beryllium has nine nucleons so it has an atomic mass of 9 and 6.02x1023 (or 1 mole of) atoms of Beryllium have a mass of 9g. |