Difference between revisions of "Distillation"
(→About Distillation) |
(→About Distillation) |
||
| Line 23: | Line 23: | ||
*[[Separating Mixtures|Separating]] two [[solute]]s from each other in [[solution]] - [[Chromatography]] | *[[Separating Mixtures|Separating]] two [[solute]]s from each other in [[solution]] - [[Chromatography]] | ||
*[[Separating Mixtures|Separating]] an [[insoluble]] [[solid]] from a [[soluble]] [[solid]] - [[Filtration]] | *[[Separating Mixtures|Separating]] an [[insoluble]] [[solid]] from a [[soluble]] [[solid]] - [[Filtration]] | ||
| + | *[[Separating Mixtures|Separating]] two [[solvent]]s from each other in [[solution]] - [[Fractional Distillation]] | ||
Revision as of 10:30, 21 January 2019
Contents
Key Stage 3
Meaning
Distillation is a method of separating mixtures that can recover both the solute and the solvent in a solution.
About Distillation
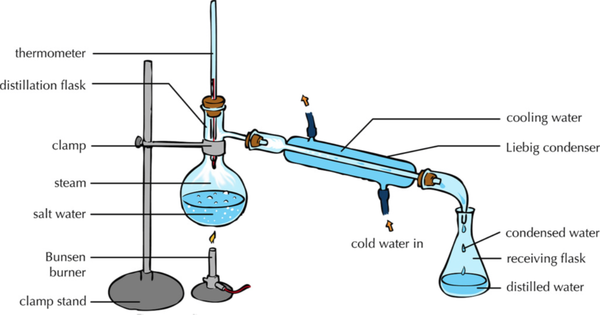
- During distillation the solvent is evaporated away to leave the solute behind, then the solvent is collected and condensed back into a liquid.
| This diagram shows how the solute and solvent can be separated by heating the solution to evaporate the solvent leaving the solute behind and then condensing the evaporated solvent by cooling it down and collecting it in a conical flask. |
Key Stage 4
Meaning
Distillation is a technique used to separate solutes and solvents from solution] that can recover both the solute and the solvent.
About Distillation
Distillation can only be used for:
- Separating a solution to recover both the solutes and solvents from the solutions.
- Separating and recovering insoluble solids from a liquid. However, this is unnecessary as it can be done by filtration.
Distillation cannot be used for:
- Separating two solutes from each other in solution - Chromatography
- Separating an insoluble solid from a soluble solid - Filtration
- Separating two solvents from each other in solution - Fractional Distillation