Difference between revisions of "Emission Spectra"
| Line 8: | Line 8: | ||
: An '''emission spectrum''' is a set of specific [[wavelength]]s with a high [[intensity]]. This appears as bright lines of [[colour]] on a [[spectrum]]. | : An '''emission spectrum''' is a set of specific [[wavelength]]s with a high [[intensity]]. This appears as bright lines of [[colour]] on a [[spectrum]]. | ||
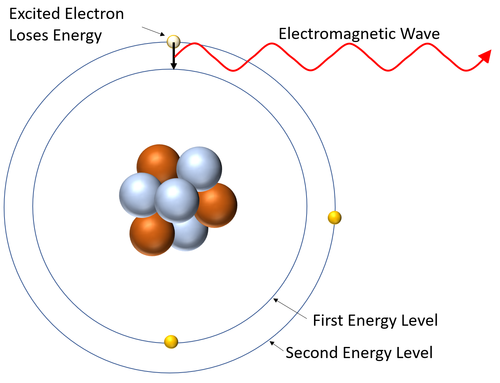
: A '''emission spectrum''' is created when [[excited]] [[electron]]s ([[electron]]s in high [[Energy Level|energy levels]]) lose [[energy]] and fall to a lower [[Energy Level|energy level]] [[emit]]ting a specific [[wavelength]] of [[electromagnetic Wave|electromagnetic wave]] when they do. | : A '''emission spectrum''' is created when [[excited]] [[electron]]s ([[electron]]s in high [[Energy Level|energy levels]]) lose [[energy]] and fall to a lower [[Energy Level|energy level]] [[emit]]ting a specific [[wavelength]] of [[electromagnetic Wave|electromagnetic wave]] when they do. | ||
| + | : The [[wavelength]]s of [[electromagnetic Wave|electromagnetic wave]] depend on the [[energy]] difference between the [[Energy Level|energy levels]] in [[atom]]s. | ||
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 13:15, 22 February 2019
Key Stage 4
Meaning
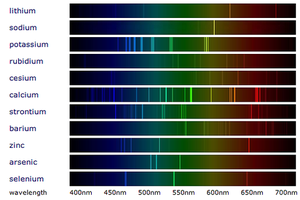
Emission spectra are the specific wavelengths of light emitted by the electrons in atoms as they lose energy.
About Emission Spectra
- The spectrum of white light is a continuous change of colours with all wavelengths having the same intensity.
- An emission spectrum is a set of specific wavelengths with a high intensity. This appears as bright lines of colour on a spectrum.
- A emission spectrum is created when excited electrons (electrons in high energy levels) lose energy and fall to a lower energy level emitting a specific wavelength of electromagnetic wave when they do.
- The wavelengths of electromagnetic wave depend on the energy difference between the energy levels in atoms.
| This diagram shows an excited electron losing energy by emitting an electromagnetic wave. As it does this the electron falls back down to a lower energy level. |