Difference between revisions of "Reactivity"
| (2 intermediate revisions by the same user not shown) | |||
| Line 57: | Line 57: | ||
*The [[Electrical Charge|charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]] but the [[electron]] shielding caused by the two inner [[electron]]s remains the same. This causes the [[electron]]s to experience a greater [[force]] of [[attraction]] as you move along the [[period]], making it easier for an [[atom]]s to gain more [[electron]]s to become [[ion]]s. | *The [[Electrical Charge|charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]] but the [[electron]] shielding caused by the two inner [[electron]]s remains the same. This causes the [[electron]]s to experience a greater [[force]] of [[attraction]] as you move along the [[period]], making it easier for an [[atom]]s to gain more [[electron]]s to become [[ion]]s. | ||
|} | |} | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Reactivity; alkali metals, pages 26-27, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Reactivity; metals, pages 26-27, 84-89, 220-221, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Reactivity; periodicity, pages 19, 30-31, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Reactivity, pages 86-87, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Reactivity; metals, pages 70-71, 125, 133, 141-143, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Reactivity; noble gases, page 137, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Reactivity; practical activities, pages 264-265, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Reactivity; trends in Periodic Table, pages 133, 135, 137, 142-143, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 17:46, 18 December 2019
Contents
Key Stage 4
Meaning
Reactivity is how vigorously a chemical will react.
About Reactivity
- Reactivity is determined by how easily an element can lose or gain electrons.
- Electrons are held in orbit around the nucleus because the electrons are negatively charged and are attracted to the nucleus which is positively charged.
- If an element loses electrons easily it is highly reactive.
- If an element gains electrons readily it is also highly reactive.
Three important factors affect reactivity of elements.
- The charge of the nucleus
- The shielding effect of inner electrons.
- Distance between the nucleus and the outer shell.
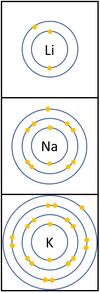
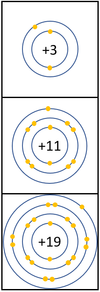
Reactivity in Groups 1, 2 and 3
| In a chemical reaction the electron in the outer shell is lost.
The reactivity increases as you go down the group because:
|
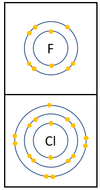
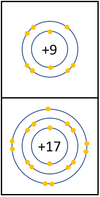
Reactivity in Group 7
| In a chemical reaction an extra electron is added to the outer shell.
The reactivity decreases as you go down the group because:
|
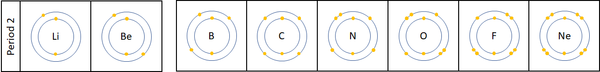
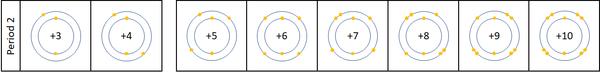
Reactivity along Period 2
| For the first 3 elements Lithium, Beryllium and Boron all lose electrons in chemical reactions.
The reactivity decreases as you go across the period because:
Nitrogen, Oxygen and Fluorine can all gain electrons to become negative ions in certain reactions. The reactivity increases as you go across the period because:
|
References
AQA
- Reactivity; alkali metals, pages 26-27, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Reactivity; metals, pages 26-27, 84-89, 220-221, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Reactivity; periodicity, pages 19, 30-31, GCSE Chemistry; Third Edition, Oxford University Press, AQA
Edexcel
OCR
- Reactivity; metals, pages 70-71, 125, 133, 141-143, Gateway GCSE Chemistry, Oxford, OCR
- Reactivity; noble gases, page 137, Gateway GCSE Chemistry, Oxford, OCR
- Reactivity; practical activities, pages 264-265, Gateway GCSE Chemistry, Oxford, OCR
- Reactivity; trends in Periodic Table, pages 133, 135, 137, 142-143, Gateway GCSE Chemistry, Oxford, OCR