Difference between revisions of "Reactivity"
| Line 35: | Line 35: | ||
The [[reactivity]] decreases as you go down the group because: | The [[reactivity]] decreases as you go down the group because: | ||
*The outer [[electron]]s are further away from the [[Atomic Nucleus|nucleus]] with each additional [[Electron Orbital|electron shell]] making the [[force]] of [[attract]]ion weaker. This makes it less able to gain an extra [[electron]]. | *The outer [[electron]]s are further away from the [[Atomic Nucleus|nucleus]] with each additional [[Electron Orbital|electron shell]] making the [[force]] of [[attract]]ion weaker. This makes it less able to gain an extra [[electron]]. | ||
| − | *Even though the [[Electrical Charge|charge]] of the [[Atomic Nucleus|nucleus]] increases the outer [[electron]]s are shielded from most of the [[Positive Charge|positive charge]] of the [[Atomic Nucleus|nucleus]] by [[electron]]s in the | + | *Even though the [[Electrical Charge|charge]] of the [[Atomic Nucleus|nucleus]] increases the outer [[electron]]s are shielded from most of the [[Positive Charge|positive charge]] of the [[Atomic Nucleus|nucleus]] by [[electron]]s in the inner shells. |
|} | |} | ||
Revision as of 10:43, 6 April 2019
Contents
Key Stage 4
Meaning
Reactivity is how vigorously a chemical will react.
About Reactivity
- Reactivity is determined by how easily an element can lose or gain electrons.
- Electrons are held in orbit around the nucleus because the electrons are negatively charged and are attracted to the nucleus which is positively charged.
- If an element loses electrons easily it is highly reactive.
- If an element gains electrons readily it is also highly reactive.
Three important factors affect reactivity of elements.
- The charge of the nucleus
- The shielding effect of inner electrons.
- Distance between the nucleus and the outer shell.
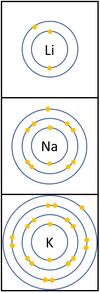
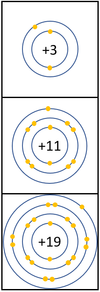
Reactivity in Groups 1, 2 and 3
| In a chemical reaction the electron in the outer shell is lost.
The reactivity increases as you go down the group because:
|
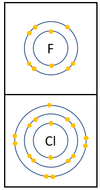
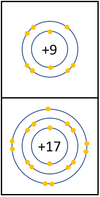
Reactivity in Group 7
| In a chemical reaction an extra electron is added to the outer shell.
The reactivity decreases as you go down the group because:
|
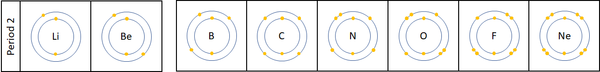
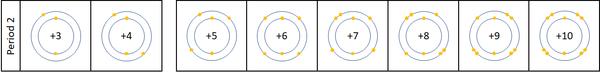
Reactivity along Period 2
| For the first 3 elements Lithium, Beryllium and Boron all lose electrons in chemical reactions.
The reactivity decreases as you go across the period because:
Nitrogen, Oxygen and Fluorine can all gain electrons to become negative ions in certain reactions. The reactivity increases as you go across the period because:
|