Difference between revisions of "Electron Shells"
(Created page with "==Key Stage 3== ===Meaning=== '''Electron shells''' are the places around a nucleus where an electron can orbit the nucleus. {| class="wikitable" |- |File:So...") |
(→About Electron Shells) |
||
| Line 14: | Line 14: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | |[[File:ElectronShells.png|center| | + | |[[File:ElectronShells.png|center|600px]] |
|- | |- | ||
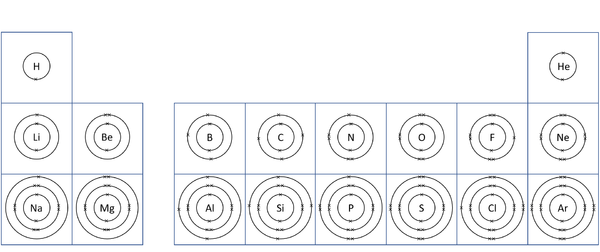
| style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the first 20 [[element]]s in the [[Periodic Table]] showing the '''electron shells'''. | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the first 20 [[element]]s in the [[Periodic Table]] showing the '''electron shells'''. | ||
|} | |} | ||
Revision as of 14:32, 1 October 2018
Key Stage 3
Meaning
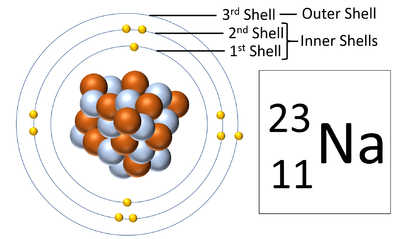
Electron shells are the places around a nucleus where an electron can orbit the nucleus.
| A diagram of a Sodium atom shown the electron shells. |
About Electron Shells
- The number of electron shells is shown by the period on the Periodic Table.
- The number of electrons in the outer shell determines the chemical properties of the element.
| A diagram of the first 20 elements in the Periodic Table showing the electron shells. |