Difference between revisions of "State Change"
| Line 25: | Line 25: | ||
===Energy and State Changes=== | ===Energy and State Changes=== | ||
| + | : Changing state either needs energy to happen or releases stored energy when it happens. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:EnergyStateChanges.png|center|500px]] | ||
| + | |- | ||
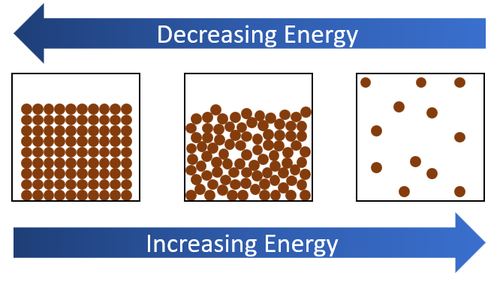
| + | | style="height:20px; width:200px; text-align:center;" |When [[energy]] is added by [[heat]]ing a [[solid]] will turn into a [[liquid]] and then a [[liquid]] will turn into a [[gas]]. When [[energy]] is taken away by cooling a [[gas]] will turn into a [[liquid]] and a [[liquid]] will turn into a [[solid]]. | ||
| + | |} | ||
| + | |||
| + | *[[Melting]], [[Evaporating]] and [[Subliming]] are [[endothermic]] changes because they need [[energy]] to happen. The [[material]] has more [[energy]] at the end than it did the beginning. | ||
| + | *[[Freezing]], [[Condensing]] and [[Depositing]] are [[exothermic]] changes because it releases [[energy]] when it happens. The [[material]] has less [[after]] it has happened. | ||
| + | |||
| + | : When [[human]]s [[sweat]] the [[water]] [[evaporation|evaporates]] from the [[skin]] helping them cool down. This works because [[evaporation]] is an [[endothermic]] process so the [[sweat]] takes [[energy]] away from the [[skin]] when it [[evaporation|evaporates]]. | ||
Revision as of 17:37, 29 September 2018
Contents
Key Stage 2
Meaning
A state change is when a material changes from one state of matter to another.
About State Changes
- A state of matter can change if the temperature changes.
- Melting is when solid turns into a liquid.
- Freezing is when a liquid turns into a solid.
- Evaporating is when a liquid turns into a gas.
- Condensing is when a gas turns into a liquid.
Key Stage 3
Meaning
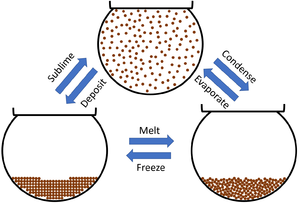
This diagram shows the changes of state between the three states of matter.
A state change is when a material changes from one state of matter to another.
About State Changes
- A state of matter can change if the temperature changes.
- Melting is when solid turns into a liquid.
- Freezing is when a liquid turns into a solid.
- Evaporating is when a liquid turns into a gas.
- Condensing is when a gas turns into a liquid.
- Subliming is when a solid turns into a gas.
- Depositing is when a gas turns into a solid.
Energy and State Changes
- Changing state either needs energy to happen or releases stored energy when it happens.
| When energy is added by heating a solid will turn into a liquid and then a liquid will turn into a gas. When energy is taken away by cooling a gas will turn into a liquid and a liquid will turn into a solid. |
- Melting, Evaporating and Subliming are endothermic changes because they need energy to happen. The material has more energy at the end than it did the beginning.
- Freezing, Condensing and Depositing are exothermic changes because it releases energy when it happens. The material has less after it has happened.
- When humans sweat the water evaporates from the skin helping them cool down. This works because evaporation is an endothermic process so the sweat takes energy away from the skin when it evaporates.