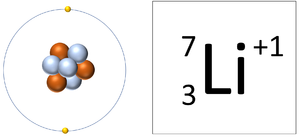Difference between revisions of "Relative Atomic Charge"
| Line 14: | Line 14: | ||
{| class="wikitable" | {| class="wikitable" | ||
|[[File:LithiumIon+Symbol.png|center|300px]] | |[[File:LithiumIon+Symbol.png|center|300px]] | ||
| + | |[[File:FluorineIon+Symbol.png|center|300px]] | ||
|- | |- | ||
| style="height:20px; width:300px; text-align:center;" |A [[Lithium]] [[ion]] has 3 [[proton]]s and 2 [[electron]]s so it has a [[Positive Charge|positive charge]]. It now has a full [[Outer Shell]]. | | style="height:20px; width:300px; text-align:center;" |A [[Lithium]] [[ion]] has 3 [[proton]]s and 2 [[electron]]s so it has a [[Positive Charge|positive charge]]. It now has a full [[Outer Shell]]. | ||
| − | |||
| − | |||
| − | |||
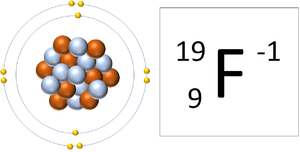
| style="height:20px; width:300px; text-align:center;" |A [[Fluorine]] [[atom]] has 9 [[proton]]s and 10 [[electron]]s so it is [[Negative Charge|negative charge]]. It now has a full [[Outer Shell]]. | | style="height:20px; width:300px; text-align:center;" |A [[Fluorine]] [[atom]] has 9 [[proton]]s and 10 [[electron]]s so it is [[Negative Charge|negative charge]]. It now has a full [[Outer Shell]]. | ||
|} | |} | ||
Revision as of 21:12, 25 November 2018
Key Stage 4
Meaning
Relative Atomic Charge is the charge of a particle compared to the charge of a single proton.
About Relative Atomic Charge
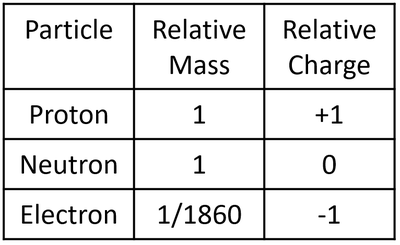
- Ions, nuclei and subatomic particles have extremely small charges such as the proton (1.6x10-19Coulombs) so instead of stating the charge in Coulombs it is compared to the charge of a proton.
| A table showing the relative mass and relative charge of the proton, neutron and electron. |
| A Lithium ion has 3 protons and 2 electrons so it has a positive charge. It now has a full Outer Shell. | A Fluorine atom has 9 protons and 10 electrons so it is negative charge. It now has a full Outer Shell. |