Difference between revisions of "Metallic Bond"
| Line 6: | Line 6: | ||
: '''Metallic bonds''' happen between [[metal]] [[atom]]s. This can be between [[atom]]s of the same [[element]] or different [[metal]] [[element]]s (an [[alloy]]). | : '''Metallic bonds''' happen between [[metal]] [[atom]]s. This can be between [[atom]]s of the same [[element]] or different [[metal]] [[element]]s (an [[alloy]]). | ||
: In '''metallic bonds''' the [[atom]]s form a [[lattice]] of [[Positive Ion|positive ions]] surrounded by a sea of [[Delocalised Electrons|delocalised electrons]]. | : In '''metallic bonds''' the [[atom]]s form a [[lattice]] of [[Positive Ion|positive ions]] surrounded by a sea of [[Delocalised Electrons|delocalised electrons]]. | ||
| + | : When [[metal]] [[atom]]s form '''metallic bonds''' they produce [[Giant Metallic Structure|giant metallic structures]] but not simple [[compound]]s. | ||
| + | : More than one [[metal]] [[element]] in a [[metal]] is referred to as an [[alloy]], not a [[compound]]. | ||
===Examples=== | ===Examples=== | ||
Revision as of 12:55, 31 December 2018
Key Stage 4
Meaning
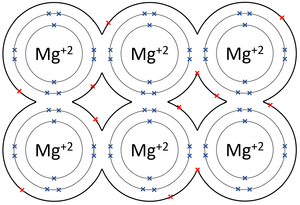
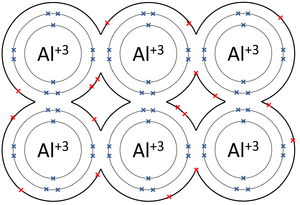
A metallic bond is type of chemical bond in which metal atoms are held together as a group of positive ions surrounded by a sea of delocalised electrons.
About Metallic Bonds
- Metallic bonds happen between metal atoms. This can be between atoms of the same element or different metal elements (an alloy).
- In metallic bonds the atoms form a lattice of positive ions surrounded by a sea of delocalised electrons.
- When metal atoms form metallic bonds they produce giant metallic structures but not simple compounds.
- More than one metal element in a metal is referred to as an alloy, not a compound.
Examples
| The outer shells of the Magnesium atoms overlap allowing the two electrons in each outer shell to move freely between atoms. | The outer shells of the Aluminium atoms overlap allowing the three electrons in each outer shell to move freely between atoms. |