Difference between revisions of "Exothermic"
(→Higher) |
(→Examples) |
||
| Line 73: | Line 73: | ||
Once the [[Chemical Reaction|reaction]] is complete 806kJ will be released. | Once the [[Chemical Reaction|reaction]] is complete 806kJ will be released. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Bond''' | ||
| + | | style="height:20px; width:150px; text-align:center;" |'''Energy in kJ/mol''' | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |H-H | ||
| + | | style="height:20px; width:150px; text-align:center;" |436 | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |N≡N | ||
| + | | style="height:20px; width:150px; text-align:center;" |941 | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |N-H | ||
| + | | style="height:20px; width:150px; text-align:center;" |391 | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:StructuralDiagramHydrogen+Nitrogen.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |In the [[Chemical Reaction|reaction]] between [[Hydrogen]] and [[Nitrogen]] the [[Chemical Bond|chemical bonds]] in the [[reactant]]s must be broken first before the [[Chemical Bond|bonds]] in the [[product]]s are formed. | ||
| + | |} | ||
| + | |||
| + | There are 3 H-H [[Chemical Bond|bonds]] and 1 N≡N [[Chemical Bond|bonds]]. | ||
| + | |||
| + | 3 x 436 + 1 x 941 = 2249kJ | ||
| + | |||
| + | Therefore 2249kJ/mol are needed to break the [[Chemical Bond|bonds]] in the [[reactant]]s. | ||
| + | |||
| + | There are 6 N-H [[Chemical Bond|bonds]]. | ||
| + | |||
| + | 6 x 391 = 2346kJ | ||
| + | |||
| + | Therefore 2346kJ/mol is released when the [[Chemical Bond|bonds]] in the [[product]]s form. | ||
| + | |||
| + | Once the [[Chemical Reaction|reaction]] is complete 97kJ will be released. | ||
Revision as of 15:34, 14 January 2019
Contents
Key Stage 3
Meaning
An exothermic process is one that gives out energy. This usually causes surroundings to increase in temperature.
About Exothermic Processes
- Most chemical reactions are exothermic with means they release energy to the environment and this is observed by an increase in temperature.
| The energy stored in the reactants is released in the chemical reaction making the material increase in temperature. The products now have less energy than the reactants. |
- Freezing, Condensing and Depositing are exothermic changes because they release energy when they happen. The material has less after they have happened. However, there is usually no increase in temperature because the material is usually cooled to change state.
Key Stage 4
Meaning
An exothermic process is one that gives out energy. This usually causes surroundings to increase in temperature.
About Exothermic Processes
Foundation
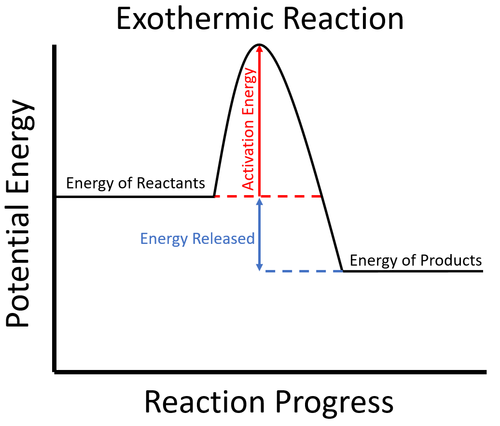
- Exothermic reactions usually require energy to begin. This is called the activation energy.
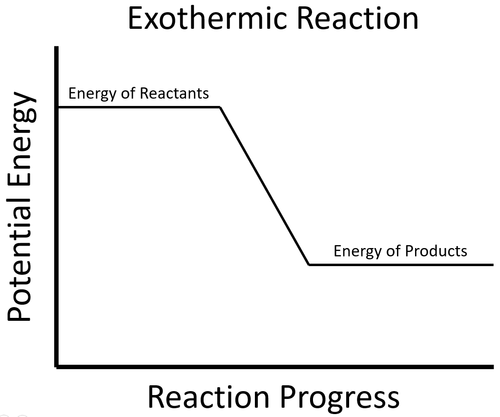
- In an exothermic process the potential energy stored in the products is less than the potential energy stored in the reactants.
| To start the reaction an activation energy is needed. This is usually achieved by initially heating the reactants. Once the reaction starts the energy stored in the reactants is released in the chemical reaction making the material increase in temperature. The products now have less energy than the reactants. |
Higher
- In a chemical reaction energy is needed to break the chemical bonds holding the atoms together.
- In an exothermic reaction energy is then released as atoms form new chemical bonds.
- This happens because there is less energy stored in the chemical bonds of the products than the reactants.
Examples
Higher
| Bond | Energy in kJ/mol |
| C-H | 413 |
| O=O | 498 |
| O-H | 464 |
| C=O | 799 |
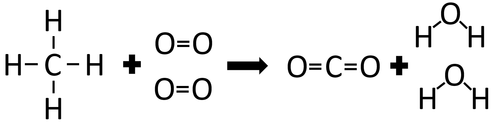
| In the reaction between Methane and Oxygen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
There are 4 C-H bonds and 2 O=O bonds.
4 x 413 + 2 x 498 = 2648kJ
Therefore 2648kJ/mol are needed to break the bonds in the reactants.
There are 2 C=O bonds and 4 O-H bonds.
2 x 799 + 4 x 464 = 3454kJ
Therefore 3454kJ/mol is released when the bonds in the products form.
Once the reaction is complete 806kJ will be released.
| Bond | Energy in kJ/mol |
| H-H | 436 |
| N≡N | 941 |
| N-H | 391 |
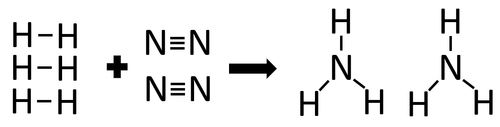
| In the reaction between Hydrogen and Nitrogen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
There are 3 H-H bonds and 1 N≡N bonds.
3 x 436 + 1 x 941 = 2249kJ
Therefore 2249kJ/mol are needed to break the bonds in the reactants.
There are 6 N-H bonds.
6 x 391 = 2346kJ
Therefore 2346kJ/mol is released when the bonds in the products form.
Once the reaction is complete 97kJ will be released.