Difference between revisions of "Absorption Spectra"
(Created page with "==Key Stage 4== ===Meaning=== right|300px|thumb|The [[Absorption Spectra|absorption spectra of several metals.]] '''Absorption spectra''' ar...") |
|||
| Line 5: | Line 5: | ||
===About Emission Spectra=== | ===About Emission Spectra=== | ||
| + | : An '''absorption spectrum''' is made by passing [[White Light|white light]] through a [[material]] or [[reflection|reflecting]] it off a [[material]] then sending it through a [[prism]] to separate the [[colour]]s. | ||
: The [[spectrum]] of [[White Light|white light]] is a continuous change of [[colour]]s with all [[wavelength]]s having the same [[intensity]]. | : The [[spectrum]] of [[White Light|white light]] is a continuous change of [[colour]]s with all [[wavelength]]s having the same [[intensity]]. | ||
: An '''absorption spectrum''' is a set of specific [[wavelength]]s with a low [[intensity]]. This appears as dark lines of of missing [[colour]] from the normal [[spectrum]] made by [[White Light|white light]]. | : An '''absorption spectrum''' is a set of specific [[wavelength]]s with a low [[intensity]]. This appears as dark lines of of missing [[colour]] from the normal [[spectrum]] made by [[White Light|white light]]. | ||
Revision as of 13:28, 22 February 2019
Key Stage 4
Meaning
File:AbsorptionSpectra.png
The absorption spectra of several metals.
Absorption spectra are the specific wavelengths of light absorbed by the electrons in atoms as they gain energy.
About Emission Spectra
- An absorption spectrum is made by passing white light through a material or reflecting it off a material then sending it through a prism to separate the colours.
- The spectrum of white light is a continuous change of colours with all wavelengths having the same intensity.
- An absorption spectrum is a set of specific wavelengths with a low intensity. This appears as dark lines of of missing colour from the normal spectrum made by white light.
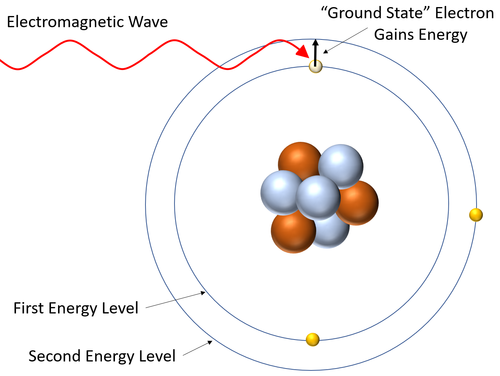
- A absorption spectrum is created when electrons absorb the energy from an electromagnetic wave and jump to a higher energy level in an atom. This removes that particular wavelength of electromagnetic wave white light being transmitted or reflected from that material.
- The wavelengths of electromagnetic wave depend on the energy difference between the energy levels in atoms.
| This diagram shows an electron gaining energy by absorbing an electromagnetic wave and becoming excited (moving to a higher energy level). |