Key Stage 4
Meaning
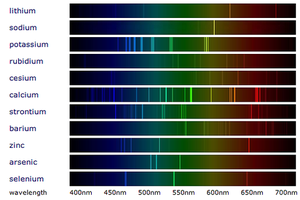
Emission spectra are the specific wavelengths of light emitted by the electrons in atoms as they lose energy.
About Emission Spectra
- The spectrum of white light is a continuous change of colours with all wavelengths having the same intensity.
- An emission spectrum is a set of specific wavelengths with a high intensity. This appears as bright lines of colour on a spectrum.
- A emission spectrum is created when excited electrons (electrons in high energy levels) lose energy and fall to a lower energy level emitting a specific wavelength of electromagnetic wave when they do.