Difference between revisions of "Chromatography"
(→About Chromatography) |
(→About Chromatography) |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
: When more than one [[solute]] is [[dissolve]]d in a [[solvent]] [[chromatography]] can be used to [[Separating Mixtures|separate]] them. | : When more than one [[solute]] is [[dissolve]]d in a [[solvent]] [[chromatography]] can be used to [[Separating Mixtures|separate]] them. | ||
: [[Chromatography]] [[experiment]]s are often done with colourful [[solute]]s which can be seen easily. | : [[Chromatography]] [[experiment]]s are often done with colourful [[solute]]s which can be seen easily. | ||
| − | + | : [[Chromatography]] works because different [[solute]]s [[diffusion|diffuse]] at different rates. | |
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 21: | Line 21: | ||
|- | |- | ||
| style="height:20px; width:500px; text-align:left;" | | | style="height:20px; width:500px; text-align:left;" | | ||
| − | + | #Draw a pencil line across a piece of [[chromatograph]] paper. | |
| − | + | #Place a dot of ink on the line. | |
| − | + | #Hang the paper so that it touches the water but do not let the ink go below the water line. | |
| − | + | #Allow the water to rise up the paper. | |
| − | + | #Once the water stops rising up the paper observe the ink to see if any colours have separated. | |
|} | |} | ||
| Line 43: | Line 43: | ||
: [[Chromatography]] relies on different [[chemical]]s experiencing different [[force]]s of [[attraction]] between the [[solvent]] used and the [[chromatography]] paper. The [[solvent]] is referred to as the '[[Mobile Phase|mobile phase]]' and acts to carry the [[chemical]] along as the [[solvent]] moves through the [[chromatography]] paper (mobile because it makes the chemical move). The [[chromatography]] paper is referred to as the '[[Stationary Phase|stationary phase]]' and acts to hold the [[chemical]]s in place (stationary because it stops them from moving). | : [[Chromatography]] relies on different [[chemical]]s experiencing different [[force]]s of [[attraction]] between the [[solvent]] used and the [[chromatography]] paper. The [[solvent]] is referred to as the '[[Mobile Phase|mobile phase]]' and acts to carry the [[chemical]] along as the [[solvent]] moves through the [[chromatography]] paper (mobile because it makes the chemical move). The [[chromatography]] paper is referred to as the '[[Stationary Phase|stationary phase]]' and acts to hold the [[chemical]]s in place (stationary because it stops them from moving). | ||
: Each [[chemical]] has a [[Retention Factor|Retention Factor (R<sub>f</sub> number)]] which is a [[ratio]] of how far the [[chemical]] moves along the paper compared to how far the [[solvent]] moves along the paper. The larger this [[Retention Factor]] the greater the [[force]] of [[attraction]] experienced by the [[chemical]] to the paper. The [[chemical]] is 'retained' in place. | : Each [[chemical]] has a [[Retention Factor|Retention Factor (R<sub>f</sub> number)]] which is a [[ratio]] of how far the [[chemical]] moves along the paper compared to how far the [[solvent]] moves along the paper. The larger this [[Retention Factor]] the greater the [[force]] of [[attraction]] experienced by the [[chemical]] to the paper. The [[chemical]] is 'retained' in place. | ||
| − | : | + | : [[Retention Factor|R<sub>f</sub>]] numbers are unique to each [[chemical]] and can be used to [[Separating Mixtures|separate]] and identify the [[chemical]]. |
| + | |||
| + | ===Method=== | ||
| + | ====Detecting Purity==== | ||
| + | #Take a piece of [[chromatography]] paper of [[width]] 2cm and [[height]] 10cm. | ||
| + | #Using a ruler draw a line with pencil across the [[width]] 1cm up from the end. | ||
| + | #Add a dot of the unknown sample on the pencil line. | ||
| + | #Suspend the [[chromatography]] paper in a suitable [[solvent]] just below the pencil line with most of the paper above the [[solvent]]. | ||
| + | #Wait until the [[solvent]] stops rising up the paper. | ||
| + | #If there is more than one dot on the [[Chromatogram]] then the [[substance]] was [[Pure|impure]]. If there is only one dot on the [[Chromatogram]] the [[substance]] was [[pure]]. | ||
| + | |||
| + | ====Identifying Chemicals by Comparison==== | ||
| + | The [[chromatogram]] from an [[experiment]] can be compared against [[chromatogram]]s of known [[chemical]]s to identify the [[chemical]]s in the original sample. | ||
| + | #Take a piece of [[chromatography]] paper of [[width]] 2cm and [[height]] 10cm. | ||
| + | #Using a ruler draw a line with pencil across the [[width]] 1cm up from the end. | ||
| + | #Add a dot of the unknown sample on the pencil line. | ||
| + | #Suspend the [[chromatography]] paper in a suitable [[solvent]] just below the pencil line with most of the paper above the [[solvent]]. | ||
| + | #Wait until the [[solvent]] stops rising up the paper. | ||
| + | #Compare this [[chromatogram]] to [[chromatogram]]s of known [[chemical]]s. | ||
| + | |||
| + | ====Identifying Chemicals by R<sub>f</sub> Number==== | ||
| + | The [[Retention Factor|R<sub>f</sub>]] values for a variety of [[chemical]]s are known. By comparing the [[Retention Factor|R<sub>f</sub>]] values from an [[experiment]] to the known values of different [[chemical]]s then the [[chemical]]s in the [[experiment]] may be identified. | ||
| + | #Take a piece of [[chromatography]] paper of [[width]] 2cm and [[height]] 10cm. | ||
| + | #Using a ruler draw a line with pencil across the [[width]] 1cm up from the end. | ||
| + | #Add a dot of the unknown sample on the pencil line. | ||
| + | #Suspend the [[chromatography]] paper in a suitable [[solvent]] just below the pencil line with most of the paper above the [[solvent]]. | ||
| + | #Wait until the [[solvent]] stops rising up the paper. | ||
| + | #Use a ruler to measure the distance that the [[solvent]] has traveled up the paper. | ||
| + | #Use a ruler to measure the distance that the [[chemical]] has traveled up the paper. | ||
| + | #Use the following equation to find the [[Retention Factor]]: | ||
| + | :<math>R_f = \frac{d_c}{d_s}</math> | ||
| + | :Where: | ||
| + | :R<sub>f</sub> = [[Retention Factor]] | ||
| + | :d<sub>c</sub> = distance moved by the [[chemical]] | ||
| + | :d<sub>s</sub> = distance moved by the [[solvent]] | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09771 ''Chromatography, pages 101, 151, 152, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Chromatography, pages 10-11, 182-183, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Chromatography, pages 137, 139, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Chromatography, pages 157-9, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d81 ''Chromatography, pages 16, 87, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Chromatography, pages 18, 265, 286-7, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Chromatography, pages 24, 205-6, 213, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Chromatography, pages 35, 36, 200-202, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Chromatography, pages 35, 36, 254-256, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Chromatography; gas-liquid, pages 270, 282, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Chromatography; paper, pages 262-3, 268-71, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Chromatography; thin layer, pages 263, 269, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Chromatography, page 152, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Chromatography, page 8, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Chromatography, pages 102, 103, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Chromatography, pages 107-111, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Chromatography, pages 39, 40, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Chromatography; paper 8-9, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Chromatography; paper, pages 152-153, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Chromatography, pages 102, 103, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Chromatography, pages 29, 30, 61, GCSE Chemistry; The Revision Guide, CGP, OCR Gateway ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Chromatography, pages 46-49, 153, 268-269, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 17:19, 23 February 2022
Contents
Key Stage 3
Meaning
Chromatography is a method used to separate and identify different solutes found in a solution.
About Chromatography
- When more than one solute is dissolved in a solvent chromatography can be used to separate them.
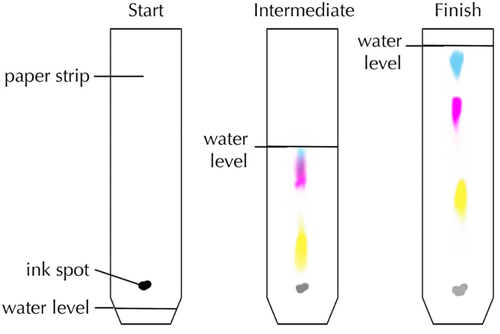
- Chromatography experiments are often done with colourful solutes which can be seen easily.
- Chromatography works because different solutes diffuse at different rates.
| This diagram shows how black ink is made of three different inks in solution. |
Method
|
Key Stage 4
Meaning
Chromatography is a technique used to separate and identify different solutes found in a solution.
About Chromatography
Chromatography can be used for:
- Separating and identifying two or more solutes from a solution.
- Identifying pure from impure substances.
Chromatography cannot be used for:
- Separating separating liquids in solution - Fractional Distillation
- Separating an insoluble solid from a soluble solid - Filtration
- Separating solutes from a solvent in solution. - Evaporation (Crystallisation) or Distillation.
- Chromatography relies on different chemicals experiencing different forces of attraction between the solvent used and the chromatography paper. The solvent is referred to as the 'mobile phase' and acts to carry the chemical along as the solvent moves through the chromatography paper (mobile because it makes the chemical move). The chromatography paper is referred to as the 'stationary phase' and acts to hold the chemicals in place (stationary because it stops them from moving).
- Each chemical has a Retention Factor (Rf number) which is a ratio of how far the chemical moves along the paper compared to how far the solvent moves along the paper. The larger this Retention Factor the greater the force of attraction experienced by the chemical to the paper. The chemical is 'retained' in place.
- Rf numbers are unique to each chemical and can be used to separate and identify the chemical.
Method
Detecting Purity
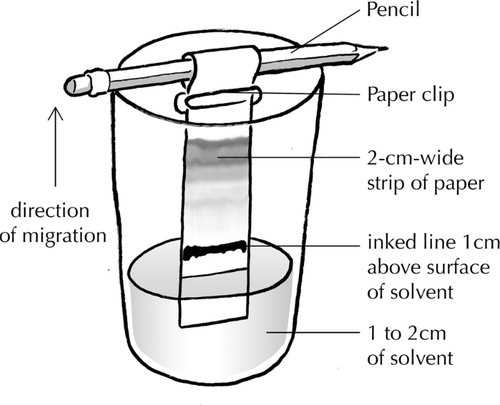
- Take a piece of chromatography paper of width 2cm and height 10cm.
- Using a ruler draw a line with pencil across the width 1cm up from the end.
- Add a dot of the unknown sample on the pencil line.
- Suspend the chromatography paper in a suitable solvent just below the pencil line with most of the paper above the solvent.
- Wait until the solvent stops rising up the paper.
- If there is more than one dot on the Chromatogram then the substance was impure. If there is only one dot on the Chromatogram the substance was pure.
Identifying Chemicals by Comparison
The chromatogram from an experiment can be compared against chromatograms of known chemicals to identify the chemicals in the original sample.
- Take a piece of chromatography paper of width 2cm and height 10cm.
- Using a ruler draw a line with pencil across the width 1cm up from the end.
- Add a dot of the unknown sample on the pencil line.
- Suspend the chromatography paper in a suitable solvent just below the pencil line with most of the paper above the solvent.
- Wait until the solvent stops rising up the paper.
- Compare this chromatogram to chromatograms of known chemicals.
Identifying Chemicals by Rf Number
The Rf values for a variety of chemicals are known. By comparing the Rf values from an experiment to the known values of different chemicals then the chemicals in the experiment may be identified.
- Take a piece of chromatography paper of width 2cm and height 10cm.
- Using a ruler draw a line with pencil across the width 1cm up from the end.
- Add a dot of the unknown sample on the pencil line.
- Suspend the chromatography paper in a suitable solvent just below the pencil line with most of the paper above the solvent.
- Wait until the solvent stops rising up the paper.
- Use a ruler to measure the distance that the solvent has traveled up the paper.
- Use a ruler to measure the distance that the chemical has traveled up the paper.
- Use the following equation to find the Retention Factor:
\[R_f = \frac{d_c}{d_s}\]
- Where:
- Rf = Retention Factor
- dc = distance moved by the chemical
- ds = distance moved by the solvent
References
AQA
- Chromatography, pages 101, 151, 152, GCSE Combined Science; The Revision Guide, CGP, AQA
- Chromatography, pages 10-11, 182-183, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Chromatography, pages 137, 139, GCSE Combined Science Trilogy 1, Hodder, AQA
- Chromatography, pages 157-9, GCSE Combined Science Trilogy 2, Hodder, AQA
- Chromatography, pages 16, 87, GCSE Chemistry; The Revision Guide, CGP, AQA
- Chromatography, pages 18, 265, 286-7, GCSE Chemistry; Student Book, Collins, AQA
- Chromatography, pages 24, 205-6, 213, GCSE Chemistry, Hodder, AQA
- Chromatography, pages 35, 36, 200-202, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Chromatography, pages 35, 36, 254-256, GCSE Chemistry, CGP, AQA
- Chromatography; gas-liquid, pages 270, 282, GCSE Chemistry; Student Book, Collins, AQA
- Chromatography; paper, pages 262-3, 268-71, GCSE Chemistry; Student Book, Collins, AQA
- Chromatography; thin layer, pages 263, 269, GCSE Chemistry; Student Book, Collins, AQA
Edexcel
- Chromatography, page 152, GCSE Combined Science, Pearson Edexcel
- Chromatography, page 8, GCSE Chemistry, Pearson, Edexcel
- Chromatography, pages 102, 103, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Chromatography, pages 107-111, GCSE Chemistry, CGP, Edexcel
- Chromatography, pages 39, 40, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Chromatography; paper 8-9, GCSE Chemistry, Pearson, Edexcel
- Chromatography; paper, pages 152-153, GCSE Combined Science, Pearson Edexcel